

Alzamend Neuro, Inc.Filed Pursuant to Rule 433 Issuer Free Writing Prospectus Registration Statement File No. 333-255955Ma¹ y 2021

FREE WRITING PROSPECTUS STATEMENT This presentation highlights basic information about us and the offering to which this presentation relates. Because it is a summary, it does not contain all of the information that you should consider before investing in our securities. Alzamend Neuro, Inc. (the “Company”) has filed a registration statement on Form S-1 (including a prospectus, which is currently in preliminary form) (File No. 333-255955) with the Securities and Exchange Commission (the “SEC”) for the offering to which this presentation relates. The registration statement has not yet become effective. Before you invest, you should read the preliminary prospectus in the registration statement (including the risk factors described therein) and other documents the Company has filed with the SEC for more complete information about the Company and this offering. You may access these documents for free by visiting EDGAR on the SEC website at www.sec.gov. The preliminary prospectus, dated May 25, 2021, is available on the SEC website at www.sec.gov/edgar. Alternatively, the Company or the underwriter participating in the offering will arrange to send you the preliminary prospectus and, when available, the final prospectus and/or any supplements thereto if you contact Spartan Capital Securities, LLC, Attention: Prospectus Department, 45 Broadway, 19th Floor, New York, NY 10006, by calling (212) 293-0123 or by e-mail at investmentbanking@spartancapital.com.MARKET, INDUSTRY AND OTHER DATA This presentation includes market and industry data and forecasts that the Company has developed from independent research reports, publicly available information, various industry publications, other published industry sources or the Company’s internal data and estimates. Independent research reports, industry publications and other published industry sources generally indicate that the information contained therein was obtained from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information. Although the Company believes that the publications and reports are reliable, the Company has not independently verified the data and makes no representation or warranty with respect to the accuracy of such information. Any and all trademarks and trade names referred to in this presentation are the property of their respective owners. The Company’s internal data, estimates and forecasts are based on information obtained from trade and business organizations and other contracts in the markets in which it operates and management’s understanding of industry conditions. Although the Company believes that such information is reliable, the Company has not had such information verified by any independent sources.2

CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS Certain statements in this presentation may constitute “forward-looking statements” within the meaning of the Private Litigation Securities Litigation Reform Act of 1995. Those statements include, but are not limited to, statements with respect to the Company’s future financial performance, our anticipated growth strategies, anticipated trends in our industry, business prospects and opportunities. These statements are generally identified by the use of words such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “aim,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential”, “continue,” “ongoing,” “target,” “seek” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These forward-looking statements may include projections about our future financial performance, growth strategies, expected product trials and approvals, and anticipated trends in our industry. All forward-looking statements speak only as of the date on which they are made. These statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions concerning future events that are difficult to predict. Therefore, actual future events or results may differ materially from these statements. Although we believe that these forward-looking statements are based on reasonable assumptions, a number of factors could cause actual results to differ materially from these statements, including, but not limited to: (i) our significant losses since inception and anticipation of continuing significant losses for the foreseeable future; (ii) our requirement of substantial additional funding to finance our operations and complete development in seeking United States Food and Drug Administration (“FDA”) approval for AL001 and AL002 before commercialization; (iii) our ability to generate revenue and achieve profitability and our ability to achieve key business strategies; (iv) our reliance on licenses from a third party regarding our rights and development of AL001 and AL002; (v) our development of AL001 and AL002 never leading to a marketable product; (vi) our AL001 and AL002 product candidates not qualifying for expedited development, or if they do, not actually leading to a faster development or regulatory review or approval process; (vii) our approach to targeting beta-amyloid plaque via AL002 being based on a novel therapeutic approach; (viii) our concentrated research and development efforts on the treatment of Alzheimer’s and other neurodegenerative diseases and psychiatric disorders, fields that have seen limited success in product development; (ix) our clinical development involving a lengthy and expensive process with an uncertain outcome; (x) our encountering substantial delays in clinical trials, or conducting or completing clinical trials on expected timelines; (xi) our significant competition with competitors which may develop and market technologies or products more rapidly or that are more effective, safer or less expensive than our product candidates; (xii) our reliance on third parties to conduct our nonclinical studies and clinical trials; and (xiii) our ability to protect our intellectual property and our proprietary technologies.Recipients are cautioned not to place undue reliance on these statements and that the foregoing may not contain all of the forward-looking statements made in this presentation. The Company does not undertake any obligation to publicly update these forward-looking statements, except as required by federal securities law.3
TABLE OF CONTENTSIntroduction Company Overview 5 Alzheimer’s Disease (“AD”) 6Overview of Alzheimer’s DiseaseOur ScienceSpecific DetailsFinal DetailsEconomic Burden 7 Therapeutic Landscape 8 General Scientific Overview 9 AL001 (LiProSal™) 10-12 AL002 (CAO22W) 13 Intellectual Property (Licensed Patents) 14 Recent IPOs of Biotechnology Companies with AD Indication 15 Use of Proceeds 16 IPO Terms 17 Our Team 18-204
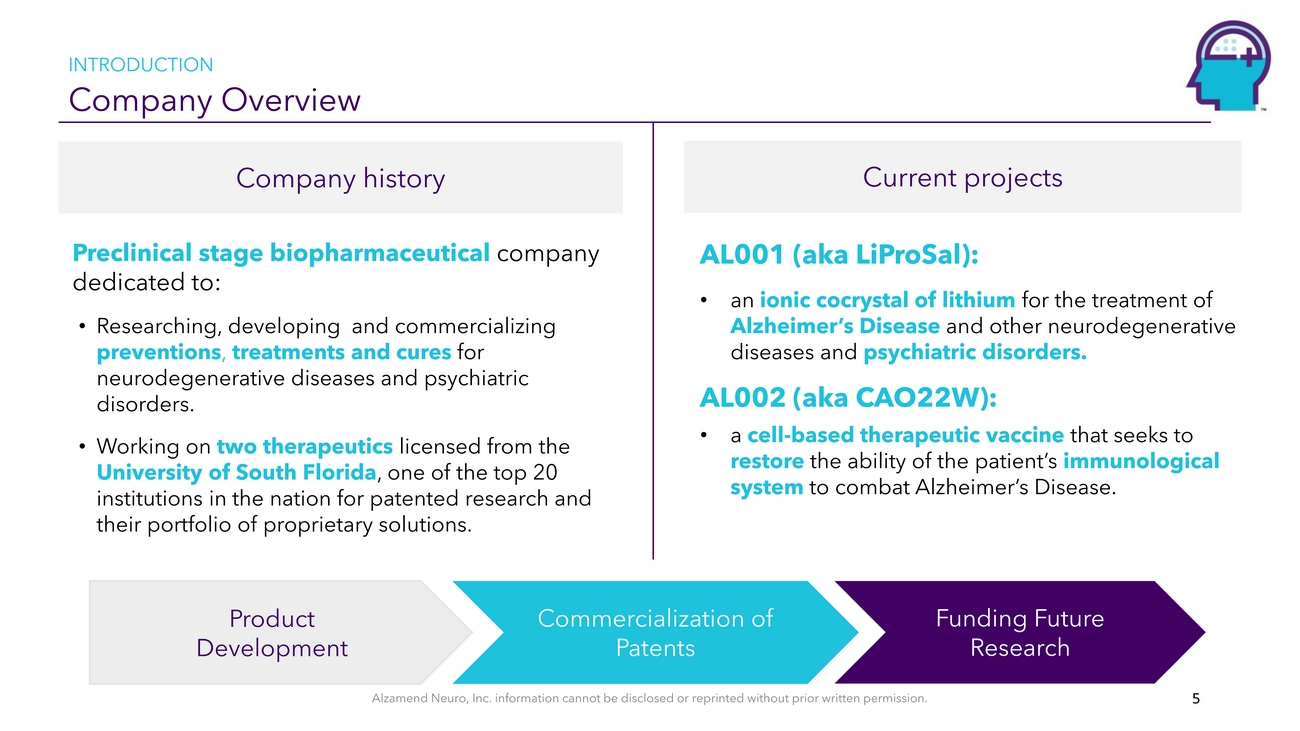
INTRODUCTION Company OverviewCompany history Current projectsPreclinical stage biopharmaceutical company dedicated to: • Researching, developing and commercializing preventions, treatments and cures for neurodegenerative diseases and psychiatric disorders. • Working on two therapeutics licensed from the University of South Florida, one of the top 20 institutions in the nation for patented research and their portfolio of proprietary solutions.AL001 (aka LiProSal): • an ionic cocrystal of lithium for the treatment of Alzheimer’s Disease and other neurodegenerative diseases and psychiatric disorders. AL002 (aka CAO22W): • a cell-based therapeutic vaccine that seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s Disease.Product DevelopmentCommercialization of PatentsFunding Future ResearchAlzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 5
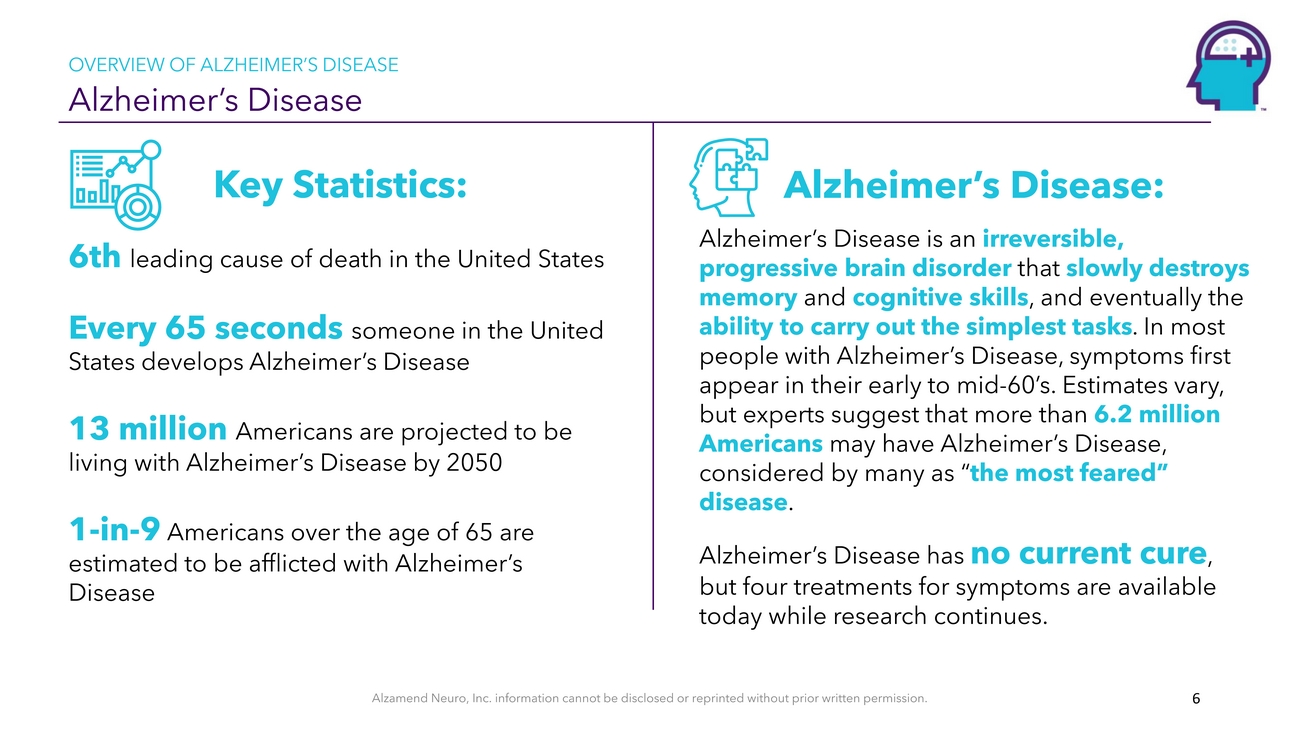
OVERVIEW OF ALZHEIMER’S DISEASE Alzheimer’s DiseaseKey Statistics: 6th leading cause of death in the United States Every 65 seconds someone in the United States develops Alzheimer’s Disease13 million Americans are projected to be living with Alzheimer’s Disease by 20501-in-9 Americans over the age of 65 are estimated to be afflicted with Alzheimer’s DiseaseAlzheimer’s Disease: Alzheimer’s Disease is an irreversible, progressive brain disorder that slowly destroys memory and cognitive skills, and eventually the ability to carry out the simplest tasks. In most people with Alzheimer’s Disease, symptoms first appear in their early to mid-60’s. Estimates vary, but experts suggest that more than 6.2 million Americans may have Alzheimer’s Disease, considered by many as “the most feared” disease. Alzheimer’s Disease has no current cure, but four treatments for symptoms are available today while research continues.Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 6
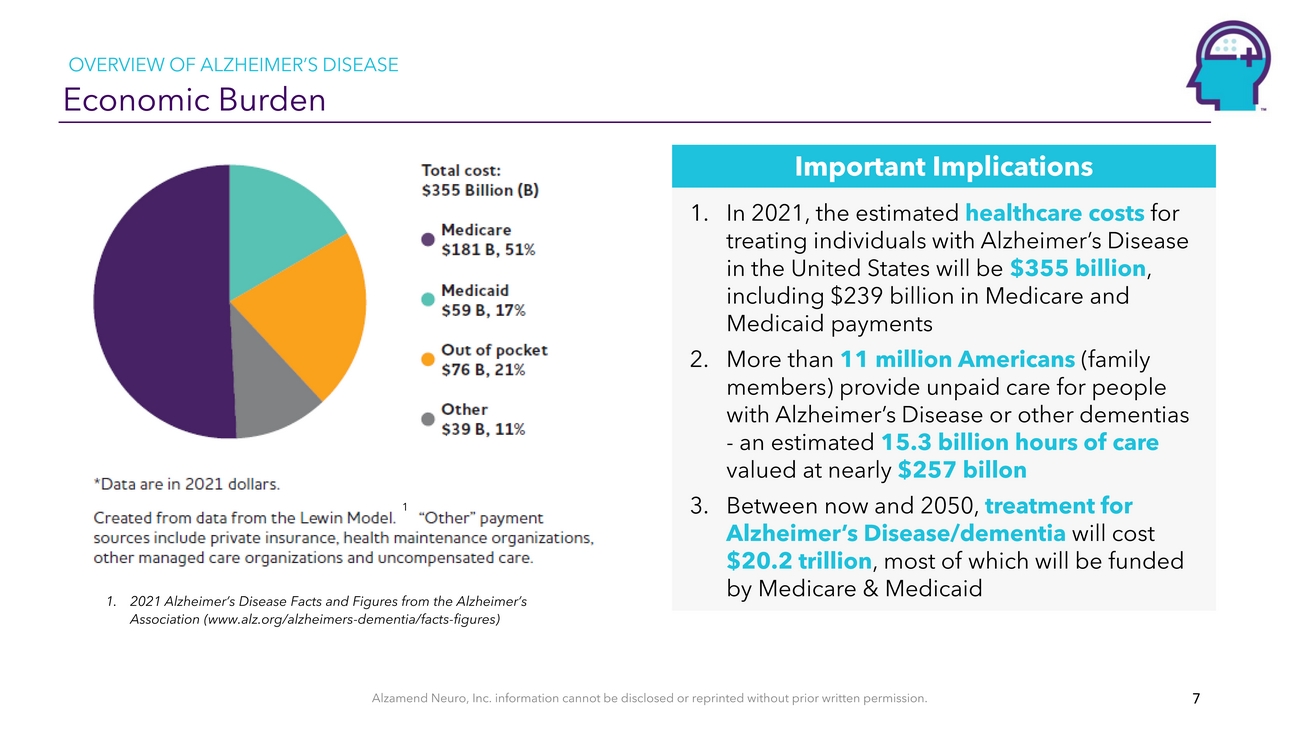
OVERVIEW OF ALZHEIMER’S DISEASE Economic Burden11. 2021 Alzheimer’s Disease Facts and Figures from the Alzheimer’s Association (www.alz.org/alzheimers-dementia/facts-figures)Important Implications 1. In 2021, the estimated healthcare costs for treating individuals with Alzheimer’s Disease in the United States will be $355 billion, including $239 billion in Medicare and Medicaid payments 2. More than 11 million Americans (family members) provide unpaid care for people with Alzheimer’s Disease or other dementias - an estimated 15.3 billion hours of care valued at nearly $257 billon 3. Between now and 2050, treatment for Alzheimer’s Disease/dementia will cost $20.2 trillion, most of which will be funded by Medicare & MedicaidAlzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 7
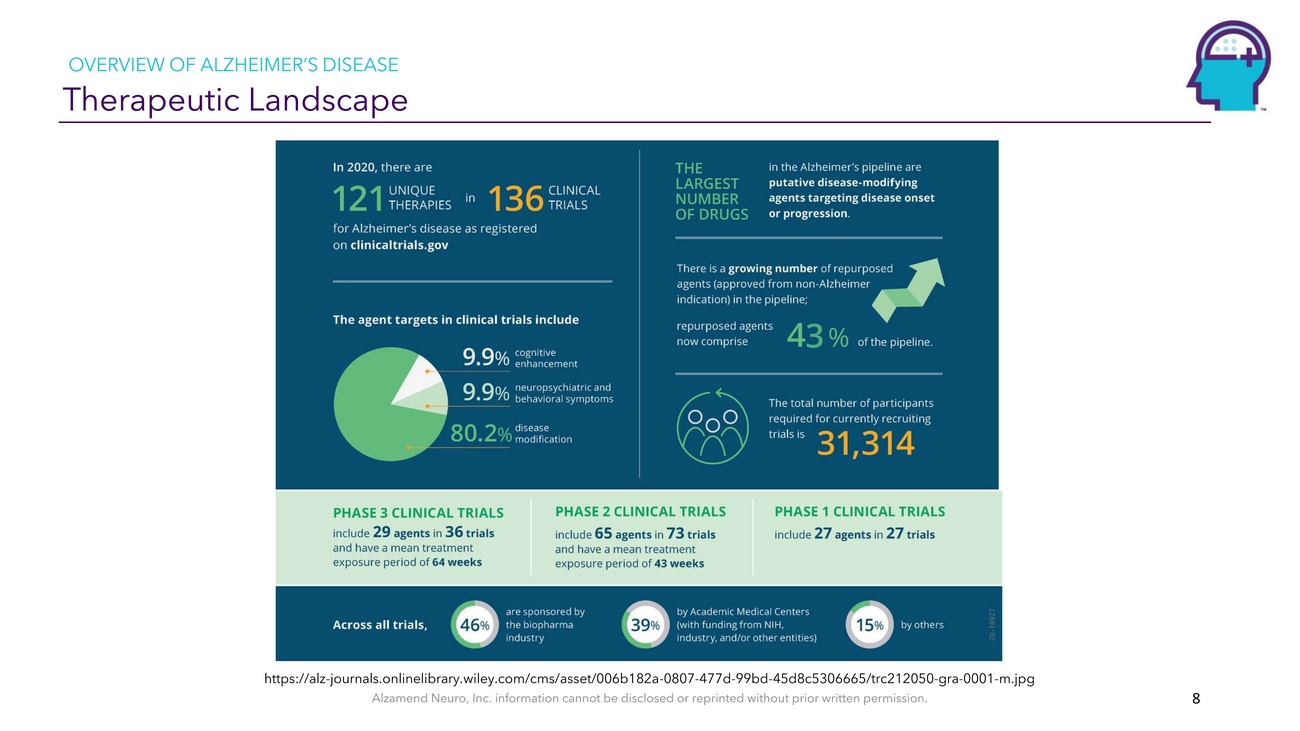
OVERVIEW OF ALZHEIMER’S DISEASE Therapeutic Landscapehttps://alz-journals.onlinelibrary.wiley.com/cms/asset/006b182a-0807-477d-99bd-45d8c5306665/trc21205 0-gra-0001-m.jpg Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 8
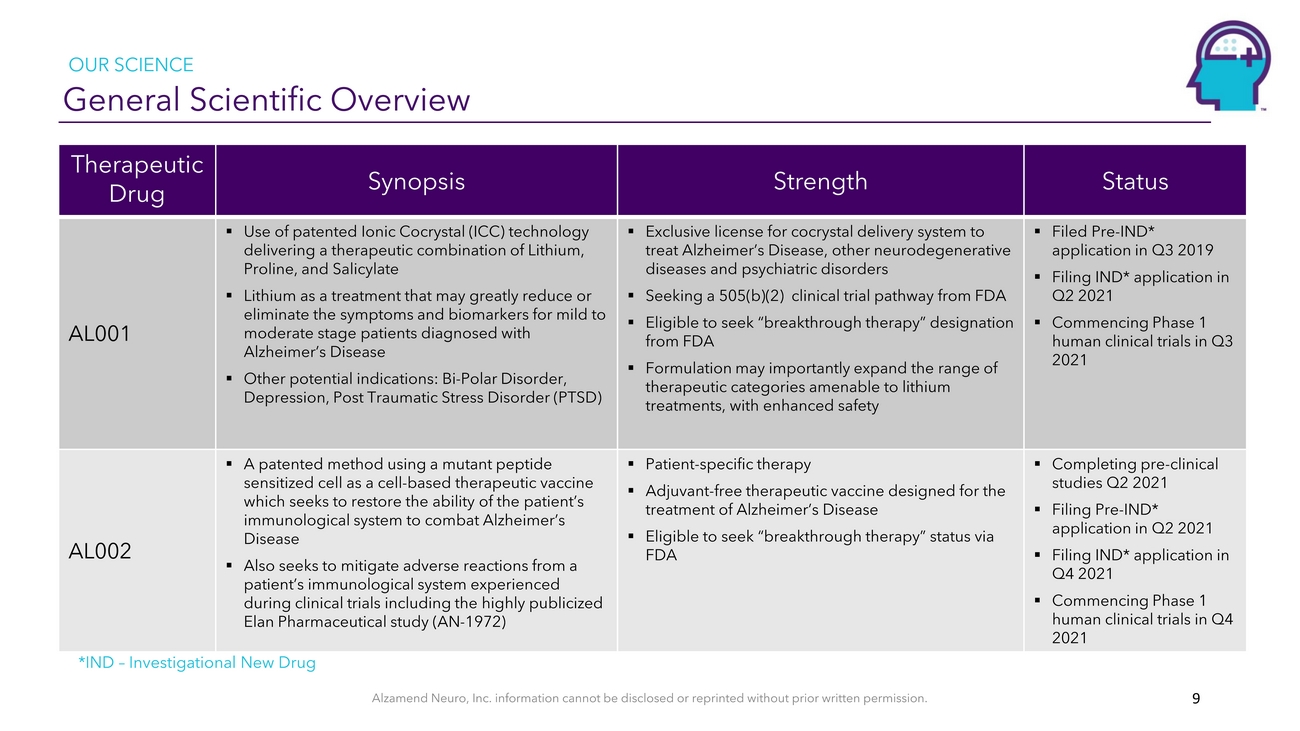
OUR SCIENCE General Scientific OverviewTherapeutic DrugAL001Synopsis▪ Use of patented Ionic Cocrystal (ICC) technology delivering a therapeutic combination of Lithium, Proline, and Salicylate ▪ Lithium as a treatment that may greatly reduce or eliminate the symptoms and biomarkers for mild to moderate stage patients diagnosed with Alzheimer’s Disease ▪ Other potential indications: Bi-Polar Disorder, Depression, Post Traumatic Stress Disorder (PTSD)Strength▪ Exclusive license for cocrystal delivery system to treat Alzheimer’s Disease, other neurodegenerative diseases and psychiatric disorders ▪ Seeking a 505(b)(2) clinical trial pathway from FDA ▪ Eligible to seek “breakthrough therapy” designation from FDA ▪ Formulation may importantly expand the range of therapeutic categories amenable to lithium treatments, with enhanced safetyStatus▪ Filed Pre-IND* application in Q3 2019 ▪ Filing IND* application in Q2 2021 ▪ Commencing Phase 1 human clinical trials in Q3 2021AL002▪ A patented method using a mutant peptide sensitized cell as a cell-based therapeutic vaccine which seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s Disease ▪ Also seeks to mitigate adverse reactions from a patient’s immunological system experienced during clinical trials including the highly publicized Elan Pharmaceutical study (AN-1972)▪ Patient-specific therapy ▪ Adjuvant-free therapeutic vaccine designed for the treatment of Alzheimer’s Disease ▪ Eligible to seek “breakthrough therapy” status via FDA▪ Completing pre-clinical studies Q2 2021 ▪ Filing Pre-IND* application in Q2 2021 ▪ Filing IND* application in Q4 2021 ▪ Commencing Phase 1 human clinical trials in Q4 2021*IND – Investigational New DrugAlzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 9
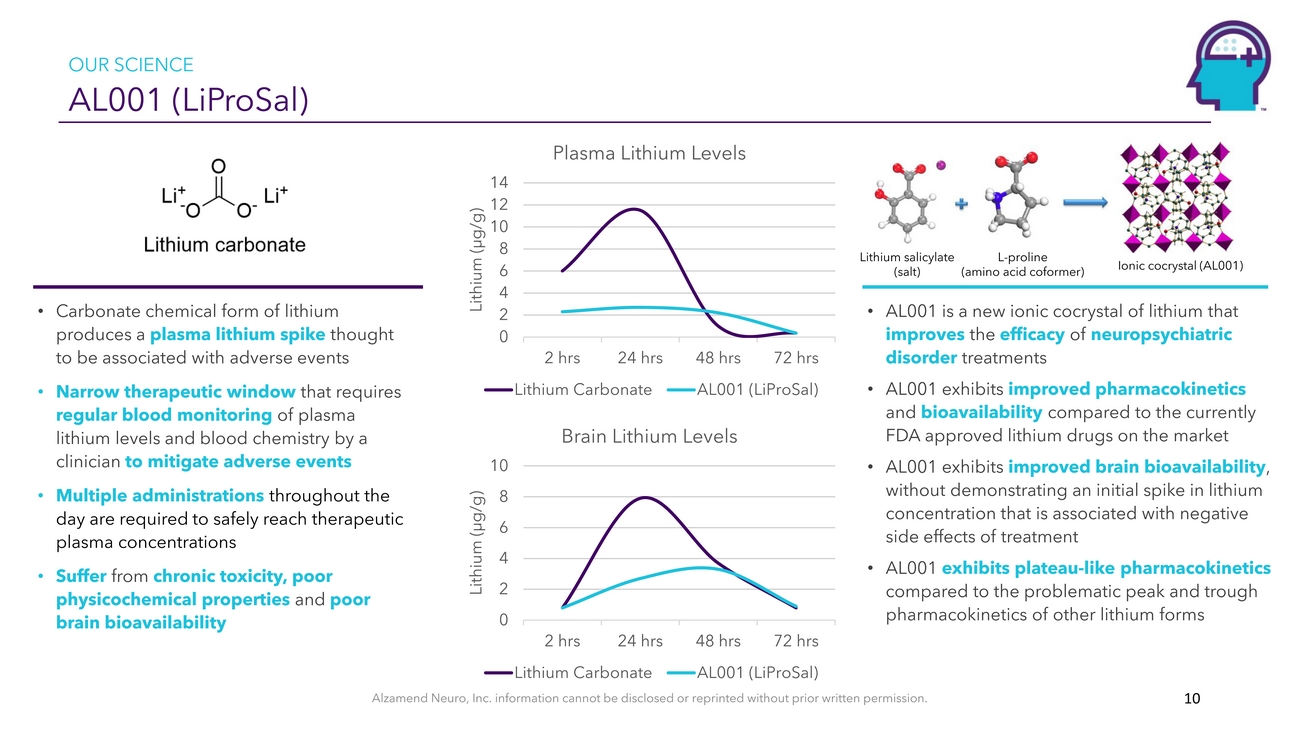
OUR SCIENCE AL001 (LiProSal)Plasma Lithium Levels 14 12 10 8 6 4Lithium salicylate (salt)L-proline (amino acid coformer)Ionic cocrystal (AL001)• Carbonate chemical form of lithium produces a plasma lithium spike thought to be associated with adverse events • Narrow therapeutic window that requires regular blood monitoring of plasma lithium levels and blood chemistry by a clinician to mitigate adverse events • Multiple administrations throughout the day are required to safely reach therapeutic plasma concentrations • Suffer from chronic toxicity, poor physicochemical properties and poor brain bioavailability2 0 2 hrs 24 hrs 48 hrs 72 hrs Lithium Carbonate AL001 (LiProSal) Brain Lithium Levels 10 8 6 4 2 0 2 hrs 24 hrs 48 hrs 72 hrs Lithium Carbonate AL001 (LiProSal)• AL001 is a new ionic cocrystal of lithium that improves the efficacy of neuropsychiatric disorder treatments • AL001 exhibits improved pharmacokinetics and bioavailability compared to the currently FDA approved lithium drugs on the market • AL001 exhibits improved brain bioavailability, without demonstrating an initial spike in lithium concentration that is associated with negative side effects of treatment • AL001 exhibits plateau-like pharmacokinetics compared to the problematic peak and trough pharmacokinetics of other lithium formsAlzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 10
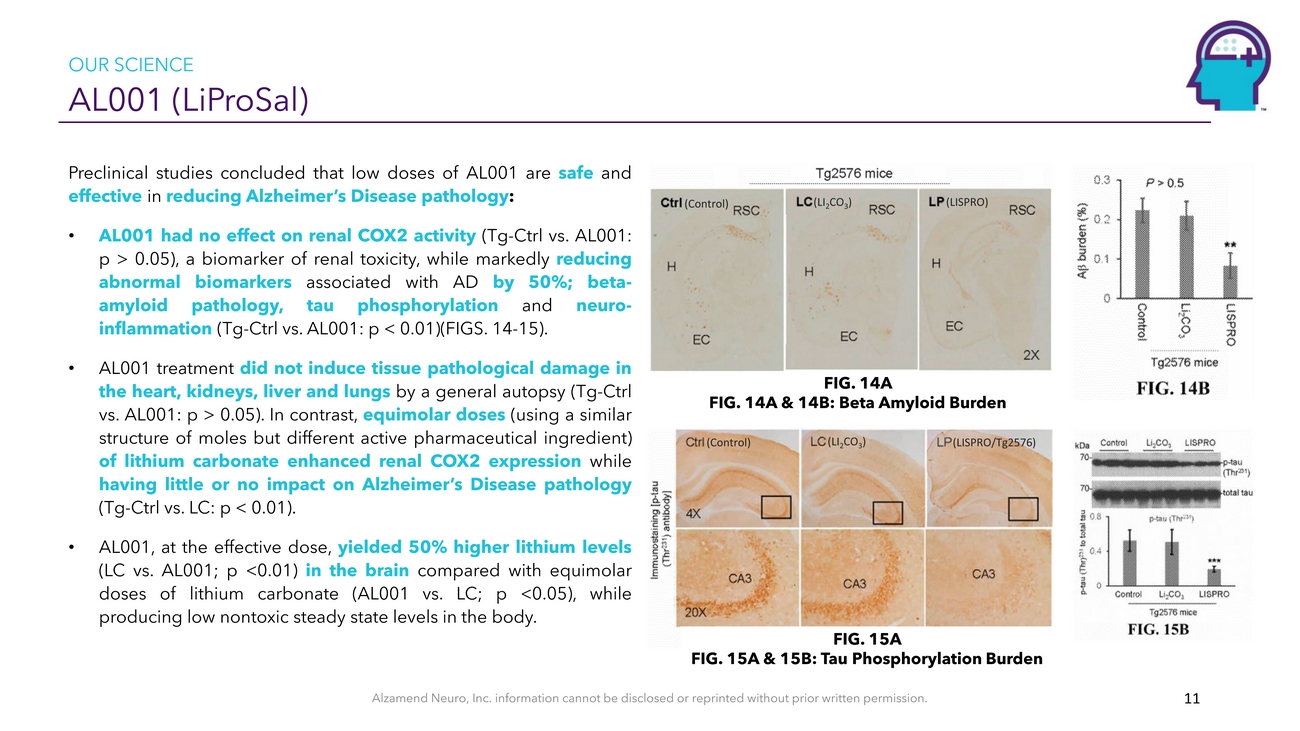
OUR SCIENCE AL001 (LiProSal)Preclinical studies concluded that low doses of AL001 are safe and effective in reducing Alzheimer’s Disease pathology:• AL001 had no effect on renal COX2 activity (Tg-Ctrl vs. AL001: p > 0.05), a biomarker of renal toxicity, while markedly reducing abnormal biomarkers associated with AD by 50%; beta- amyloid pathology, tau phosphorylation and neuro- inflammation (Tg-Ctrl vs. AL001: p < 0.01)(FIGS. 14-15). • AL001 treatment did not induce tissue pathological damage in the heart, kidneys, liver and lungs by a general autopsy (Tg-Ctrl(Control)(LI2CO3)FIG. 14A(LISPRO)vs. AL001: p > 0.05). In contrast, equimolar doses (using a similarFIG. 14A & 14B: Beta Amyloid Burdenstructure of moles but different active pharmaceutical ingredient) of lithium carbonate enhanced renal COX2 expression while having little or no impact on Alzheimer’s Disease pathology (Tg-Ctrl vs. LC: p < 0.01). • AL001, at the effective dose, yielded 50% higher lithium levels (LC vs. AL001; p <0.01) in the brain compared with equimolar doses of lithium carbonate (AL001 vs. LC; p <0.05), while producing low nontoxic steady state levels in the body.(Control)(LI₂CO₃) (LISPRO/Tg2576)FIG. 15AFIG. 15A & 15B: Tau Phosphorylation BurdenAlzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 11
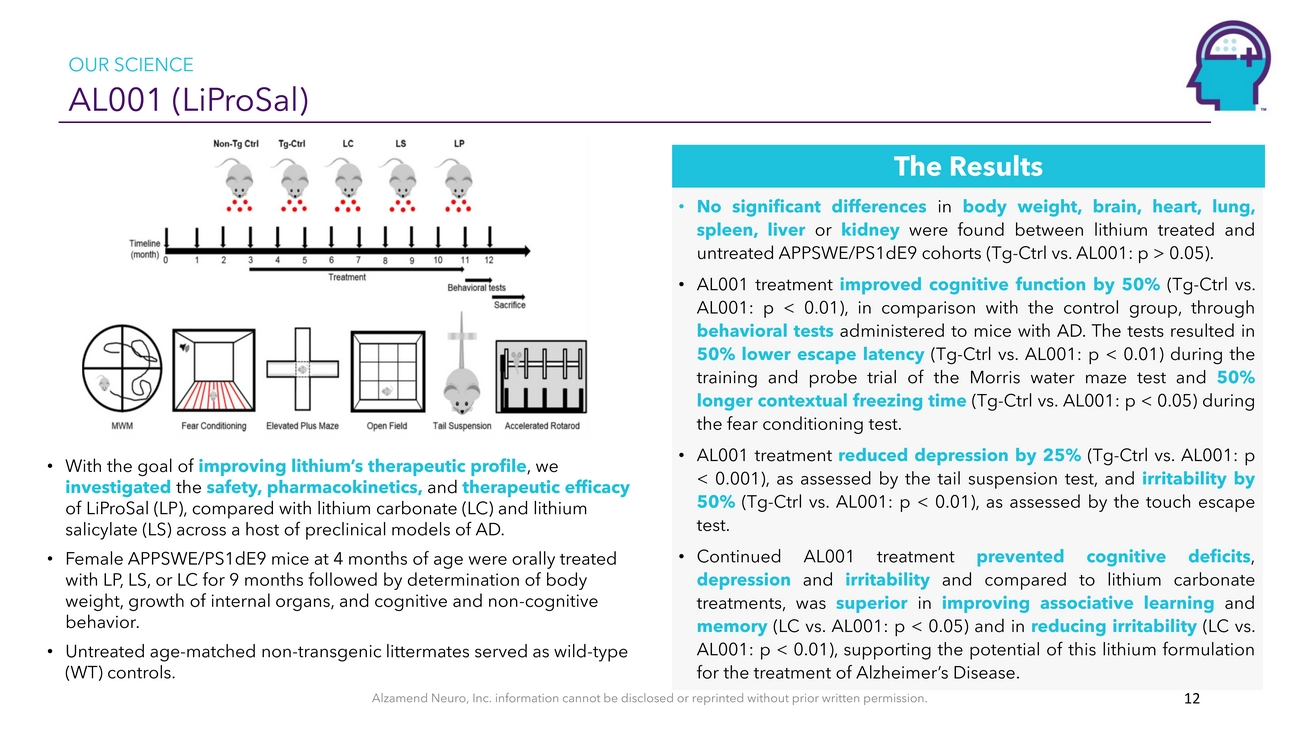
OUR SCIENCE AL001 (LiProSal)• With the goal of improving lithium’s therapeutic profile, we investigated the safety, pharmacokinetics, and therapeutic efficacy of LiProSal (LP), compared with lithium carbonate (LC) and lithium salicylate (LS) across a host of preclinical models of AD. • Female APPSWE/PS1dE9 mice at 4 months of age were orally treated with LP, LS, or LC for 9 months followed by determination of body weight, growth of internal organs, and cognitive and non-cognitive behavior. • Untreated age-matched non-transgenic littermates served as wild-type (WT) controls.The Results • No significant differences in body weight, brain, heart, lung, spleen, liver or kidney were found between lithium treated and untreated APPSWE/PS1dE9 cohorts (Tg-Ctrl vs. AL001: p > 0.05). • AL001 treatment improved cognitive function by 50% (Tg-Ctrl vs. AL001: p < 0.01), in comparison with the control group, through behavioral tests administered to mice with AD. The tests resulted in 50% lower escape latency (Tg-Ctrl vs. AL001: p < 0.01) during the training and probe trial of the Morris water maze test and 50% longer contextual freezing time (Tg-Ctrl vs. AL001: p < 0.05) during the fear conditioning test. • AL001 treatment reduced depression by 25% (Tg-Ctrl vs. AL001: p < 0.001), as assessed by the tail suspension test, and irritability by 50% (Tg-Ctrl vs. AL001: p < 0.01), as assessed by the touch escape test. • Continued AL001 treatment prevented cognitive deficits, depression and irritability and compared to lithium carbonate treatments, was superior in improving associative learning and memory (LC vs. AL001: p < 0.05) and in reducing irritability (LC vs. AL001: p < 0.01), supporting the potential of this lithium formulation for the treatment of Alzheimer’s Disease.Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 12
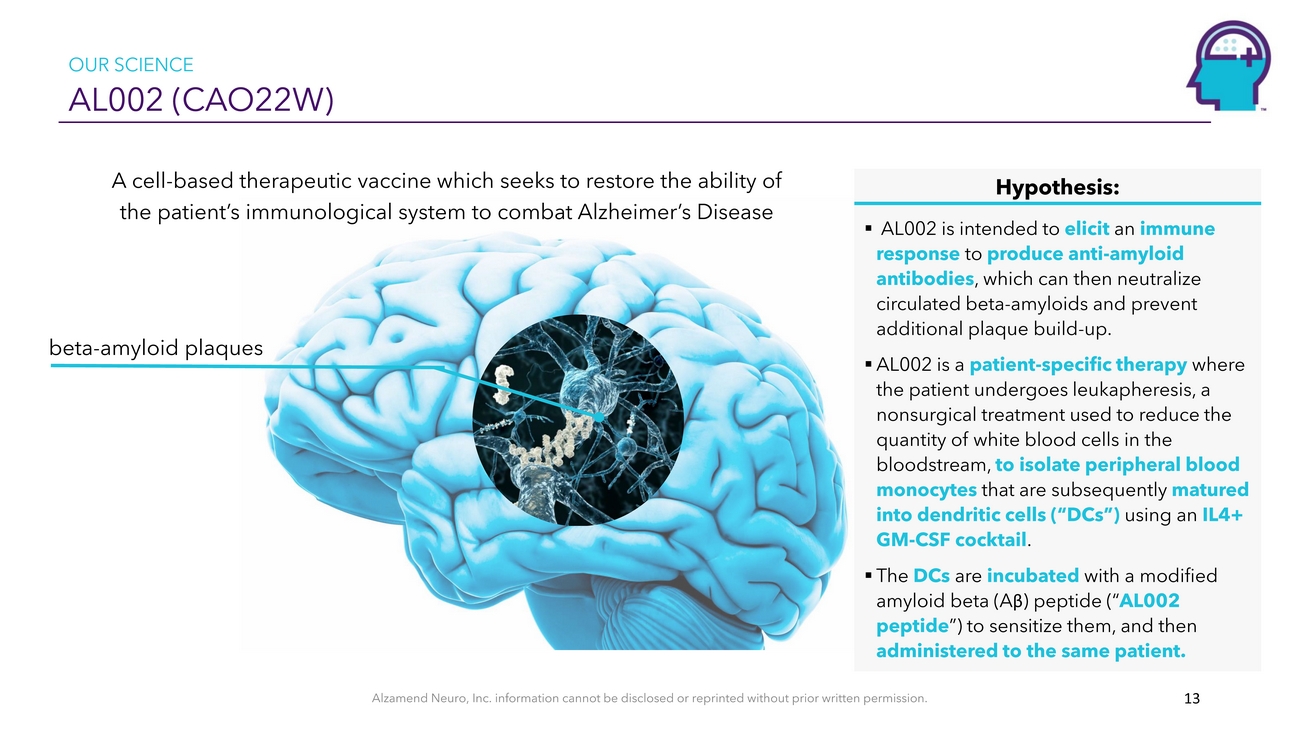
OUR SCIENCE AL002 (CAO22W)A cell-based therapeutic vaccine which seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s Diseasebeta-amyloid plaquesHypothesis: ▪ AL002 is intended to elicit an immune response to produce anti-amyloid antibodies, which can then neutralize circulated beta-amyloids and prevent additional plaque build-up. ▪ AL002 is a patient-specific therapy where the patient undergoes leukapheresis, a nonsurgical treatment used to reduce the quantity of white blood cells in the bloodstream, to isolate peripheral blood monocytes that are subsequently matured into dendritic cells (“DCs”) using an IL4+ GM-CSF cocktail. ▪ The DCs are incubated with a modified amyloid beta (Aβ) peptide (“AL002 peptide”) to sensitize them, and then administered to the same patient.Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 13
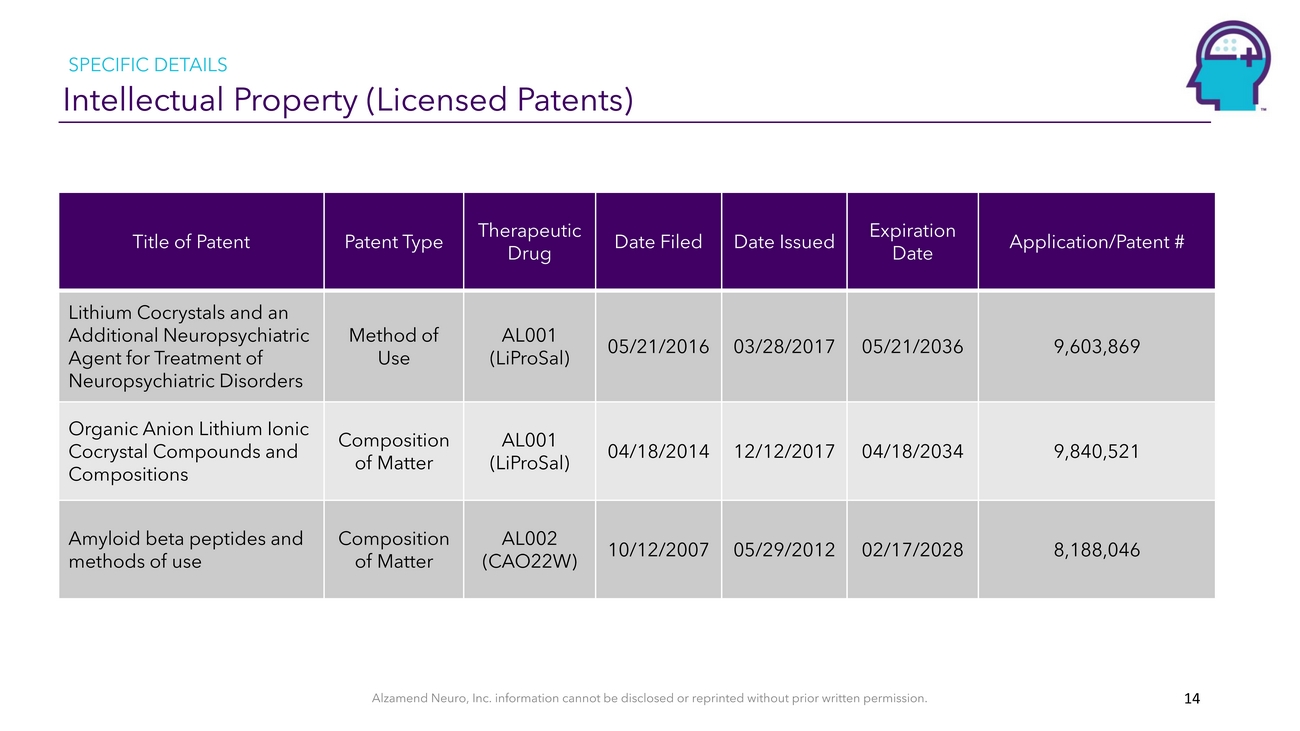
SPECIFIC DETAILS Intellectual Property (Licensed Patents)Title of PatentPatent TypeTherapeutic DrugDate FiledDate IssuedExpiration DateApplication/Patent #Lithium Cocrystals and an Additional Neuropsychiatric Agent for Treatment of Neuropsychiatric DisordersOrganic Anion Lithium Ionic Cocrystal Compounds and CompositionsMethod of UseComposition of MatterAL001 (LiProSal)AL001 (LiProSal)05/21/201604/18/201403/28/201712/12/201705/21/203604/18/20349,603,8699,840,521Amyloid beta peptides and methods of useComposition of MatterAL002 (CAO22W)10/12/200705/29/201202/17/20288,188,046Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 14
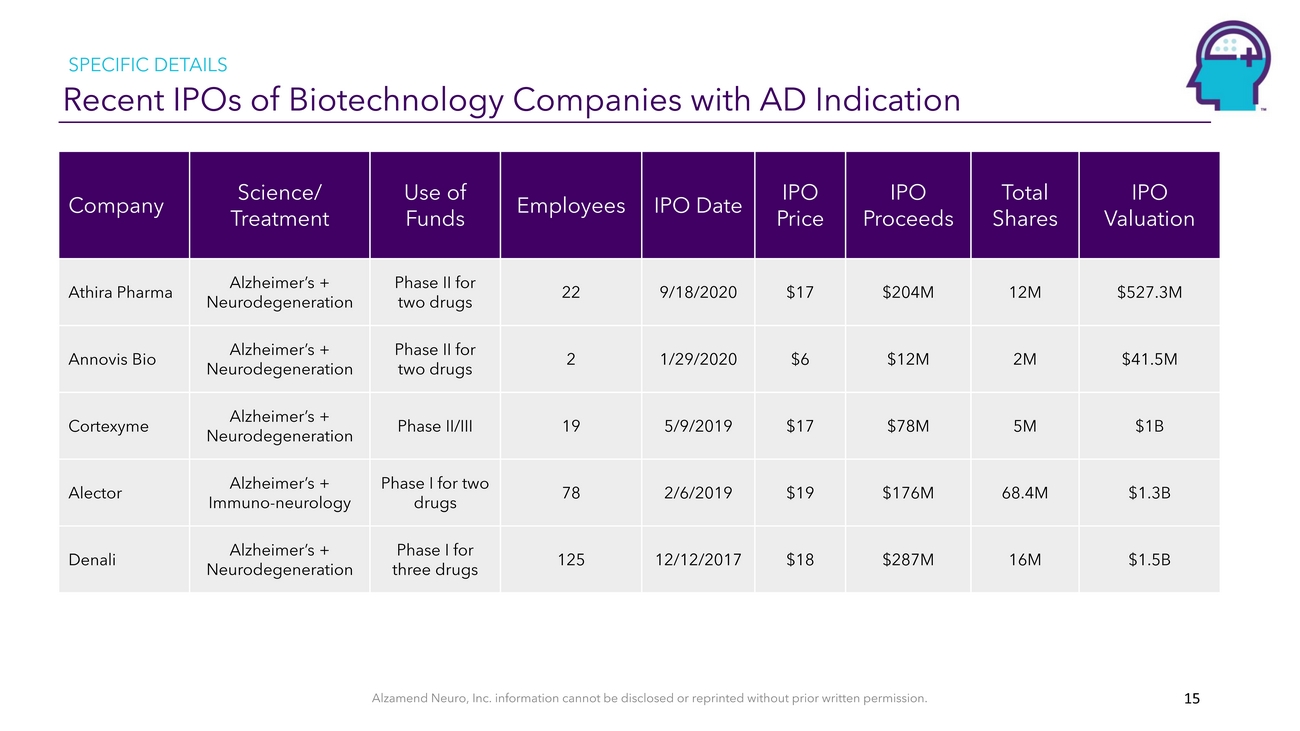
SPECIFIC DETAILS Recent IPOs of Biotechnology Companies with AD IndicationCompanyScience/ TreatmentUse of FundsEmployeesIPO DateIPO PriceIPO ProceedsTotal SharesIPO ValuationAthira PharmaAnnovis BioCortexymeAlectorDenaliAlzheimer’s + NeurodegenerationAlzheimer’s + NeurodegenerationAlzheimer’s + NeurodegenerationAlzheimer’s + Immuno-neurologyAlzheimer’s + NeurodegenerationPhase II for two drugsPhase II for two drugsPhase II/IIIPhase I for two drugsPhase I for three drugs22219781259/18/20201/29/20205/9/20192/6/201912/12/2017$17$6$17$19$18$204M$12M$78M$176M$287M12M2M5M68.4M16M$527.3M$41.5M$1B$1.3B$1.5BAlzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 15
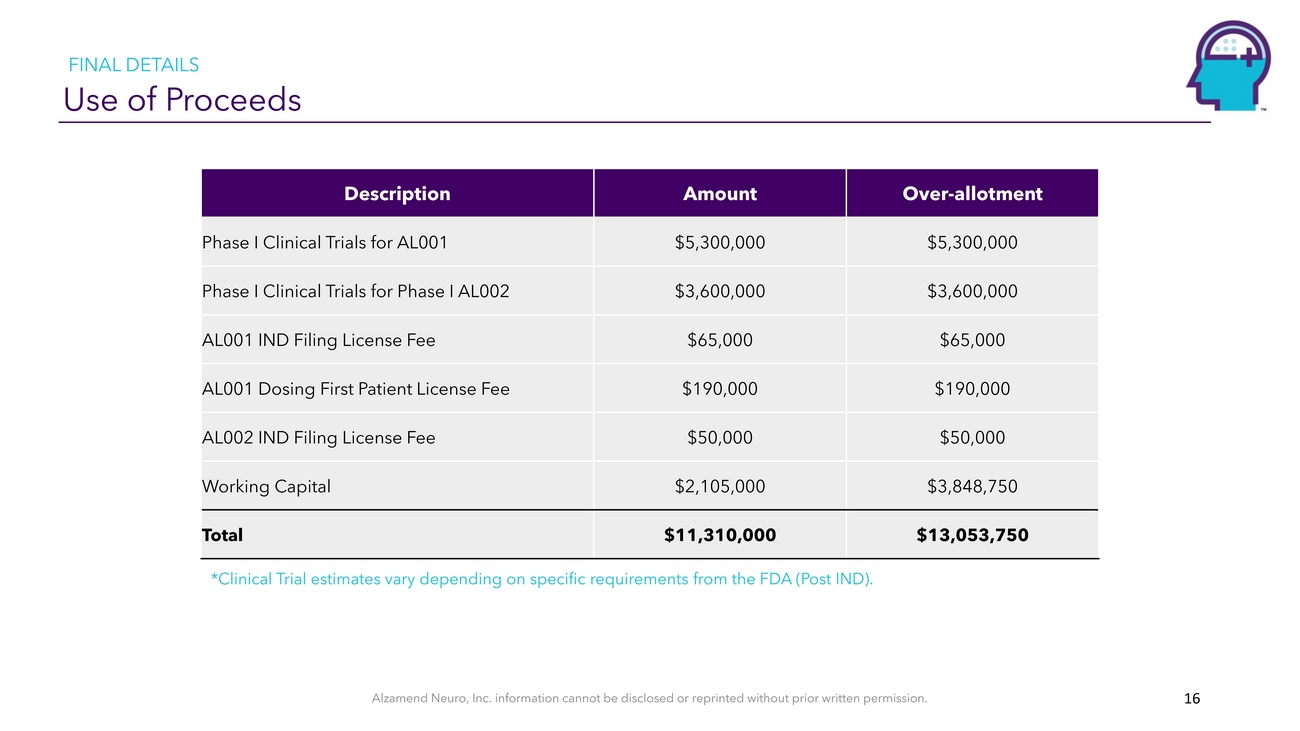
FINAL DETAILS Use of ProceedsDescriptionAmountOver-allotmentPhase I Clinical Trials for AL001$5,300,000$5,300,000Phase I Clinical Trials for Phase I AL002$3,600,000$3,600,000AL001 IND Filing License Fee$65,000$65,000AL001 Dosing First Patient License Fee$190,000$190,000AL002 IND Filing License Fee$50,000$50,000Working Capital$2,105,000$3,848,750Total$11,310,000$13,053,750*Clinical Trial estimates vary depending on specific requirements from the FDA (Post IND).Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 16
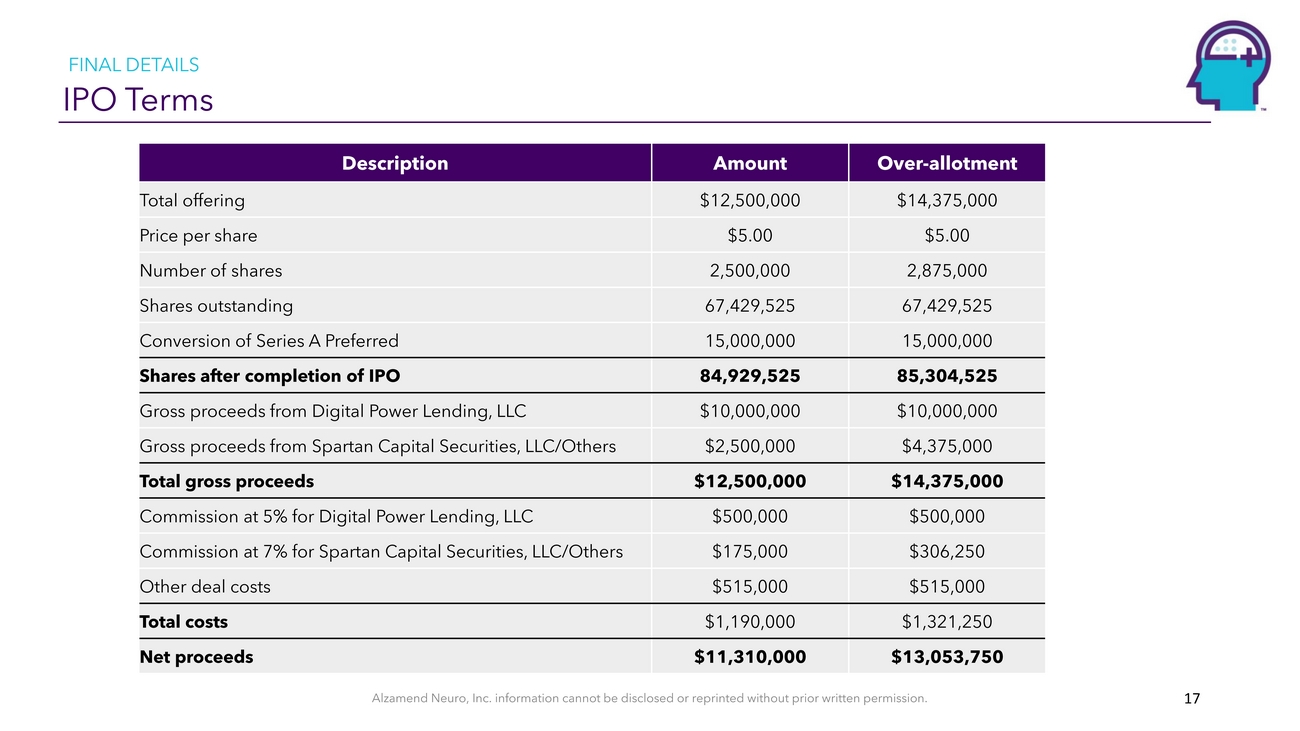
FINAL DETAILS IPO TermsTotal offering Price per share Number of shares Shares outstandingDescriptionAmount $12,500,000 $5.00 2,500,000 67,429,525Over-allotment $14,375,000 $5.00 2,875,000 67,429,525Conversion of Series A Preferred Shares after completion of IPO Gross proceeds from Digital Power Lending, LLC Gross proceeds from Spartan Capital Securities, LLC/Others Total gross proceeds Commission at 5% for Digital Power Lending, LLC Commission at 7% for Spartan Capital Securities, LLC/Others Other deal costs Total costs Net proceeds15,000,000 84,929,525 $10,000,000 $2,500,000 $12,500,000 $500,000 $175,000 $515,000 $1,190,000 $11,310,00015,000,000 85,304,525 $10,000,000 $4,375,000 $14,375,000 $500,000 $306,250 $515,000 $1,321,250 $13,053,750Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 17

FINAL DETAILS Alzamend Leadership TeamStephan Jackman Chief Executive Officer and Director 20+ years multi-industry experience, specialized in Biotech and PharmaceuticalKenneth S. Cragun Chief Financial Officer 30+ Years SEC reporting, CFO of publicly- traded company on Nasdaq, multi-industry experience, including Biotech and HealthcareDavid J. Katzoff Chief Operating Officer 30+ Years multi-industry experience, including Healthcare and TechnologyHenry Nisser Executive Vice President, General Counsel, and Director 20+ years experience, U.S. securities compliance, M&A, equity/debt financings and corporate governanceAlzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 18

FINAL DETAILS Alzamend Board of DirectorsWilliam B. Horne Chairman of Alzamend Chief Executive Officer of Ault Global Holding 25+ years Financial Industry experience, prior “Big 4” auditor and healthcare executiveStephan Jackman Chief Executive Officer and Director 20+ years multi-industry experience, specialized in Biotech and PharmaceuticalHenry Nisser Executive Vice President, General Counsel, and Director 20+ years experience, U.S. securities compliance, M&A, equity/debt financings and corporate governanceJeffrey Oram Principal at Godby Realtors 25+ years multi-industry experience, Investments, Real Estate and TechnologyAndrew H. Woo M.D., Ph.D. Practicing physician at Santa Monica Neurological Consultants, Assistant Clinical Professor of Neurology at the David Geffen School of Medicine at UCLA and Cedars-Sinai Medical Center 20+ years experience in Psychiatry and NeurologyMark Gustafson C.P.A. Chief Financial Officer of PharmaKure Limited 30+ years multi-industry experience as an active CPA, specialized in Biotech, Energy and TechnologyMilton “Todd” Ault, III Founder/Chairman Emeritus of Alzamend Chairman & CEO of Ault Global Holdings 27+ years Financial Industry experience, seasoned Wall Street CEO & activist investorAlzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 19

FINAL DETAILS Alzamend Scientific Advisory BoardEric McDade, DO Associate Director of the Dominantly Inherited Alzheimer Network Trials Unit (“DIAN-TU”), Washington University School of Medicine 76+ Peer-Reviewed Journal Publications Leads a Research Laboratory Continuously Funded by the National Institutes of Health for 10+ YearsThomas M. Wisniewski, MD Director, NYU Langone’s Pearl I. Barlow Center for Memory Evaluation and Treatment 300+ Peer-Reviewed Medical Journal Publications (28 U.S. Patents Issued) Leads a Research Laboratory Continuously Funded by the National Institutes of Health for 30+ YearsAlzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. 20

For more information please contact: Stephan Jackman Chief Executive Officer sjackman@alzamend.comFiled Pursuant to Rule 433 Issuer Free Writing Prospectus Registration Statement File No. 333-255955M²a¹ y 2021