| 1 Alzamend Neuro, Inc. June 2021 Filed Pursuant to Rule 433 Issuer Free Writing Prospectus Registration Statement File No. 333-255955 |
FREE WRITING PROSPECTUS STATEMENT
This presentation highlights basic information about us and the offering to which this presentation relates. Because it is a summary, it does not contain all of the information that you should consider before investing in our securities. Alzamend Neuro, Inc. (the “Company”) has filed a registration statement on Form S-1 (including a prospectus, which is currently in preliminary form) (File No. 333-255955) with the Securities and Exchange Commission (the “SEC”) for the offering to which this presentation relates. The registration statement has not yet become effective. Before you invest, you should read the preliminary prospectus in the registration statement (including the risk factors described therein) and other documents the Company has filed with the SEC for more complete information about the Company and this offering. You may access these documents for free by visiting EDGAR on the SEC website at www.sec.gov. The preliminary prospectus, dated June 7, 2021, is available on the SEC website at www.sec.gov/edgar. Alternatively, the Company or the underwriter participating in the offering will arrange to send you the preliminary prospectus and, when available, the final prospectus and/or any supplements thereto if you contact Spartan Capital Securities, LLC, Attention: Prospectus Department, 45 Broadway, 19th Floor, New York, NY 10006, by calling (212) 293-0123 or by e-mail at investmentbanking@spartancapital.com.
MARKET, INDUSTRY AND OTHER DATA
This presentation includes market and industry data and forecasts that the Company has developed from independent research reports, publicly available information, various industry publications, other published industry sources or the Company’s internal data and estimates. Independent research reports, industry publications and other published industry sources generally indicate that the information contained therein was obtained from sources believed to be reliable, but do not guarantee the accuracy and completeness of such information. Although the Company believes that the publications and reports are reliable, the Company has not independently verified the data and makes no representation or warranty with respect to the accuracy of such information. Any and all trademarks and trade names referred to in this presentation are the property of their respective owners. The Company’s internal data, estimates and forecasts are based on information obtained from trade and business organizations and other contracts in the markets in which it operates and management’s understanding of industry conditions. Although the Company believes that such information is reliable, the Company has not had such information verified by any independent sources.
2
|  |
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS 3 Certain statements in this presentation may constitute “forward-looking statements” within the meaning of the Private Litigation Securities Litigation Reform Act of 1995. Those statements include, but are not limited to, statements with respect to the Company’s future financial performance, our anticipated growth strategies, anticipated trends in our industry, business prospects and opportunities. These statements are generally identified by the use of words such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “aim,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing,” “target,” “seek” or the negative of these terms, or other comparable terminology intended to identify statements about the future. These forward-looking statements may include projections about our future financial performance, growth strategies, expected product trials and approvals, and anticipated trends in our industry. All forward-looking statements speak only as of the date on which they are made. These statements are not guarantees of future performance and involve certain risks, uncertainties and assumptions concerning future events that are difficult to predict. Therefore, actual future events or results may differ materially from these statements. Although we believe that these forward-looking statements are based on reasonable assumptions, a number of factors could cause actual results to differ materially from these statements, including, but not limited to:(i) our significant losses since inception and anticipation of continuing significant losses for the foreseeable future;(ii) our requirement of substantial additional funding to finance our operations and complete development in seeking United States Food and Drug Administration (“FDA”) approval for AL001 and AL002 before commercialization;(iii) our ability to generate revenue and achieve profitability and our ability to achieve key business strategies;(iv) our reliance on licenses from a third party regarding our rights and development of AL001 and AL002;(v) our development of AL001 and AL002 never leading to a marketable product;(vi) our AL001 and AL002 product candidates not qualifying for expedited development, or if they do, not actually leading to a faster development or regulatory review or approval process;(vii) our approach to targeting beta-amyloid plaque via AL002 being based on a novel therapeutic approach;(viii) our concentrated research and development efforts on the treatment of Alzheimer’s and other neurodegenerative diseases and psychiatric disorders, fields that have seen limited success in product development;(ix) our clinical development involving a lengthy and expensive process with an uncertain outcome;(x) our encountering substantial delays in clinical trials, or conducting or completing clinical trials on expected timelines;(xi) our significant competition with competitors which may develop and market technologies or products more rapidly or that are more effective, safer or less expensive than our product candidates;(xii) our reliance on third parties to conduct our nonclinical studies and clinical trials; and (xiii) our ability to protect our intellectual property and our proprietary technologies. Recipients are cautioned not to place undue reliance on these statements and that the foregoing may not contain all of the forward-looking statements made in this presentation. The Company does not undertake any obligation to publicly update these forward-looking statements, except as required by federal securities law. |
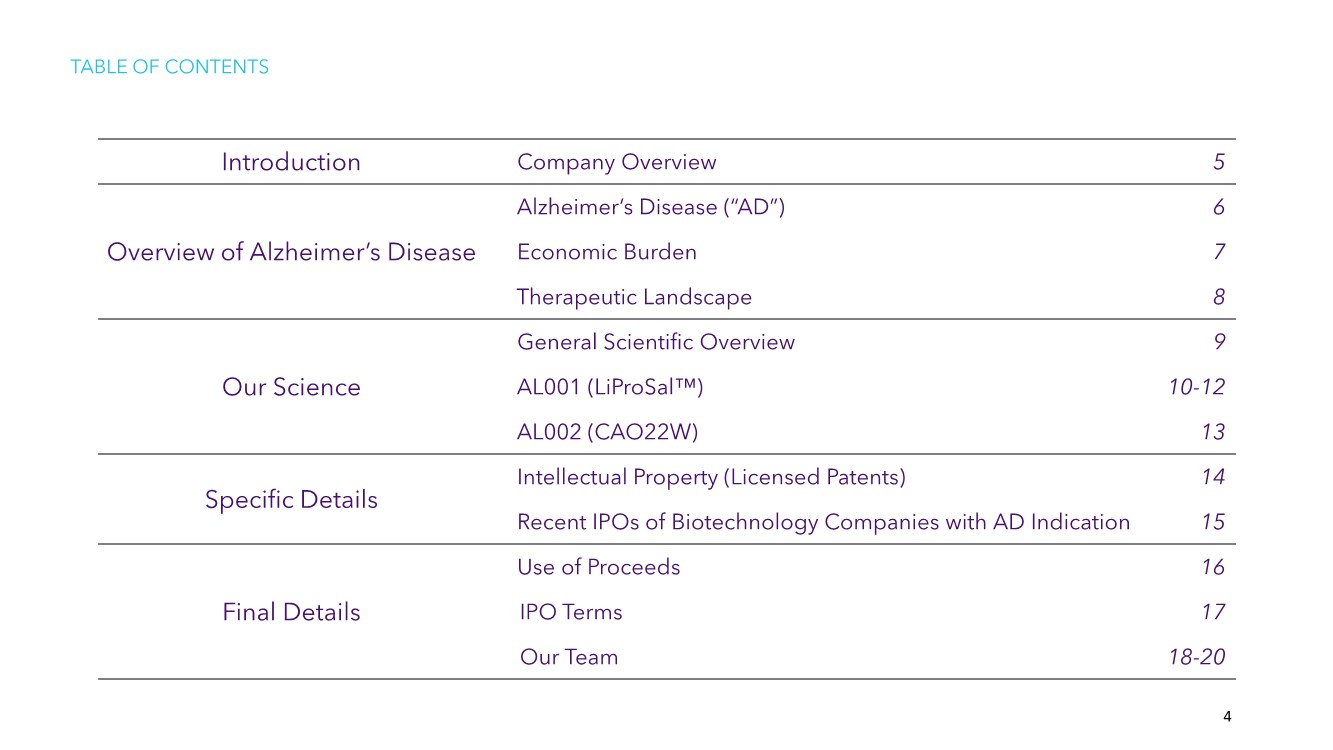
| TABLE OF CONTENTS 4 Introduction Company Overview 5 Overview of Alzheimer’s Disease Alzheimer’s Disease (“AD”) 6 Economic Burden 7 Therapeutic Landscape 8 Our Science General Scientific Overview 9 AL001 (LiProSal™) 10-12 AL002 (CAO22W) 13 Specific Details Intellectual Property (Licensed Patents) 14 Recent IPOs of Biotechnology Companies with AD Indication 15 Final Details Use of Proceeds 16 IPO Terms 17 Our Team 18-20 |
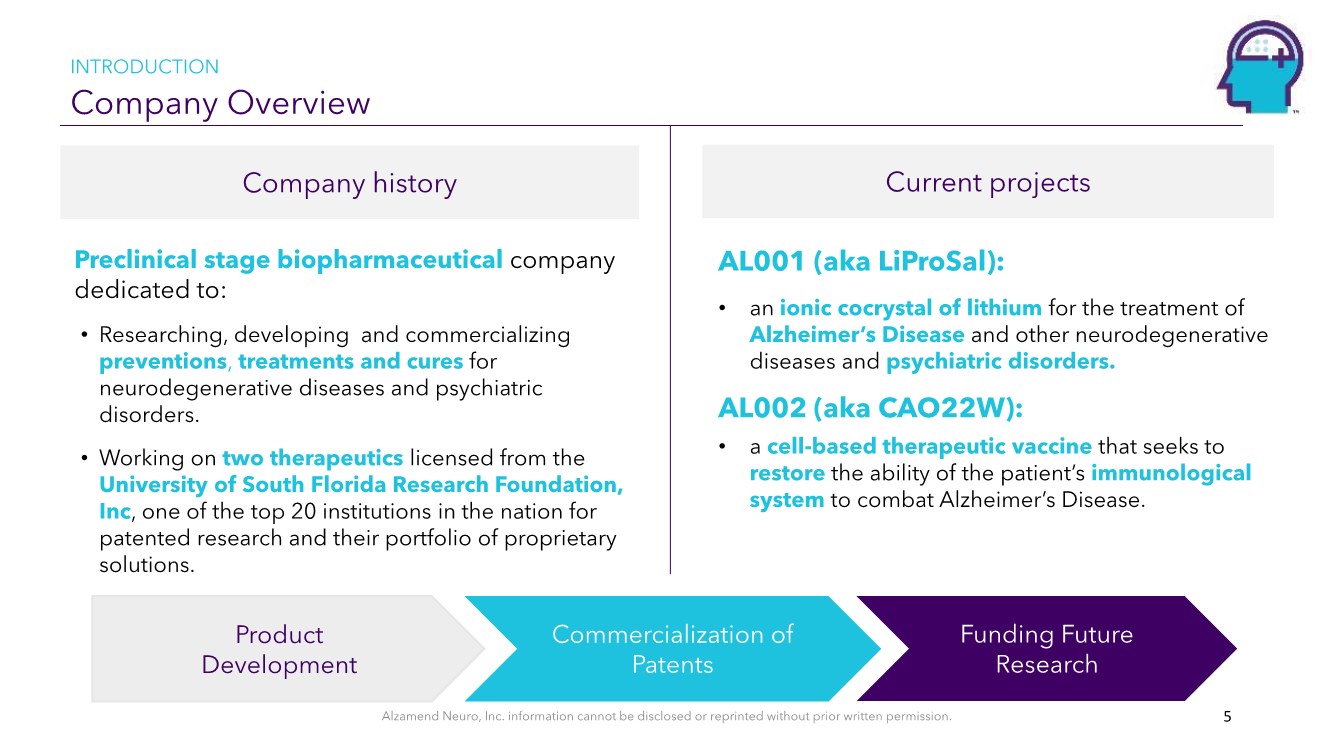
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Company Overview INTRODUCTION Company history Current projects 5 AL001 (aka LiProSal): • an ionic cocrystal of lithium for the treatment of Alzheimer’s Disease and other neurodegenerative diseases and psychiatric disorders. AL002 (aka CAO22W): • a cell-based therapeutic vaccine that seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s Disease. Preclinical stage biopharmaceutical company dedicated to: • Researching, developing and commercializing preventions, treatments and cures for neurodegenerative diseases and psychiatric disorders. • Working on two therapeutics licensed from the University of South Florida Research Foundation, Inc, one of the top 20 institutions in the nation for patented research and their portfolio of proprietary solutions. Commercialization of Patents Funding Future Research Product Development |
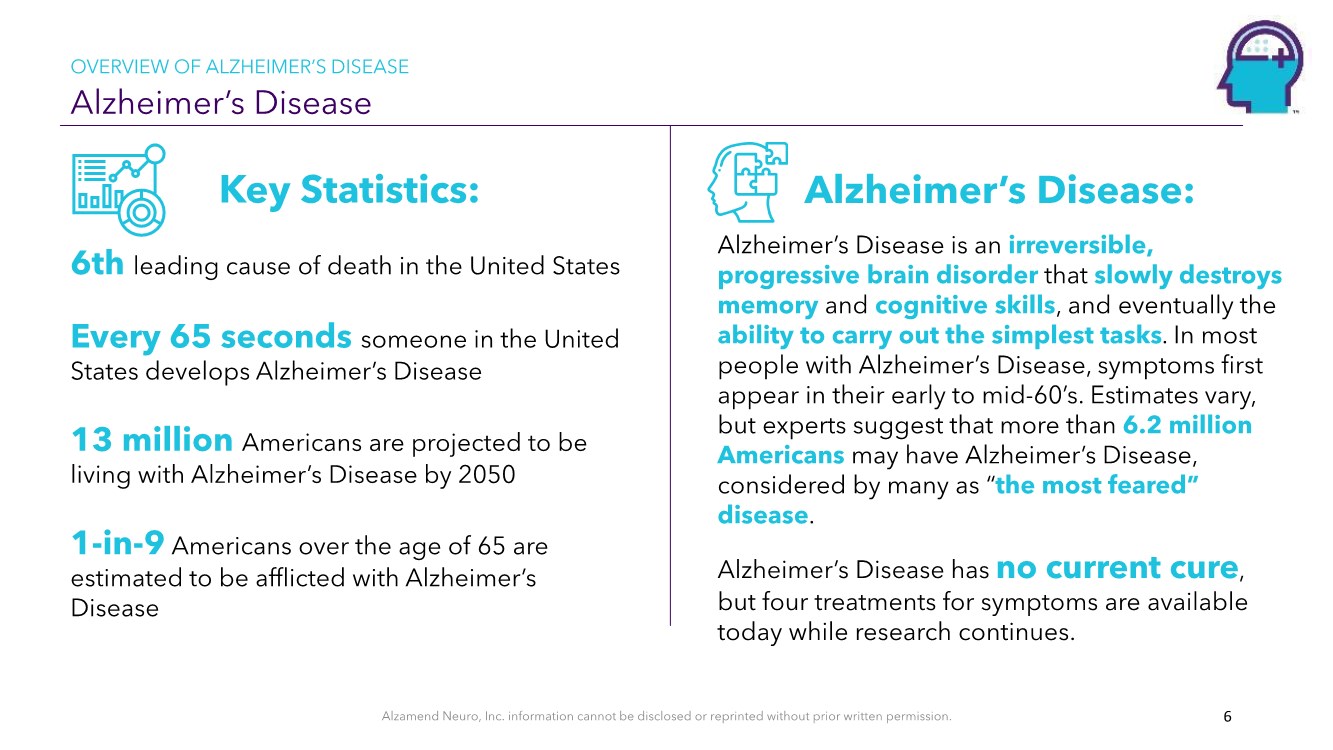
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzheimer’s Disease OVERVIEW OF ALZHEIMER’S DISEASE 6 Alzheimer’s Disease: Alzheimer’s Disease is an irreversible, progressive brain disorder that slowly destroys memory and cognitive skills, and eventually the ability to carry out the simplest tasks. In most people with Alzheimer’s Disease, symptoms first appear in their early to mid-60’s. Estimates vary, but experts suggest that more than 6.2 million Americans may have Alzheimer’s Disease, considered by many as “the most feared” disease. Alzheimer’s Disease has no current cure, but four treatments for symptoms are available today while research continues. Key Statistics: 6th leading cause of death in the United States Every 65 seconds someone in the United States develops Alzheimer’s Disease 13 million Americans are projected to be living with Alzheimer’s Disease by 2050 1-in-9 Americans over the age of 65 are estimated to be afflicted with Alzheimer’s Disease |
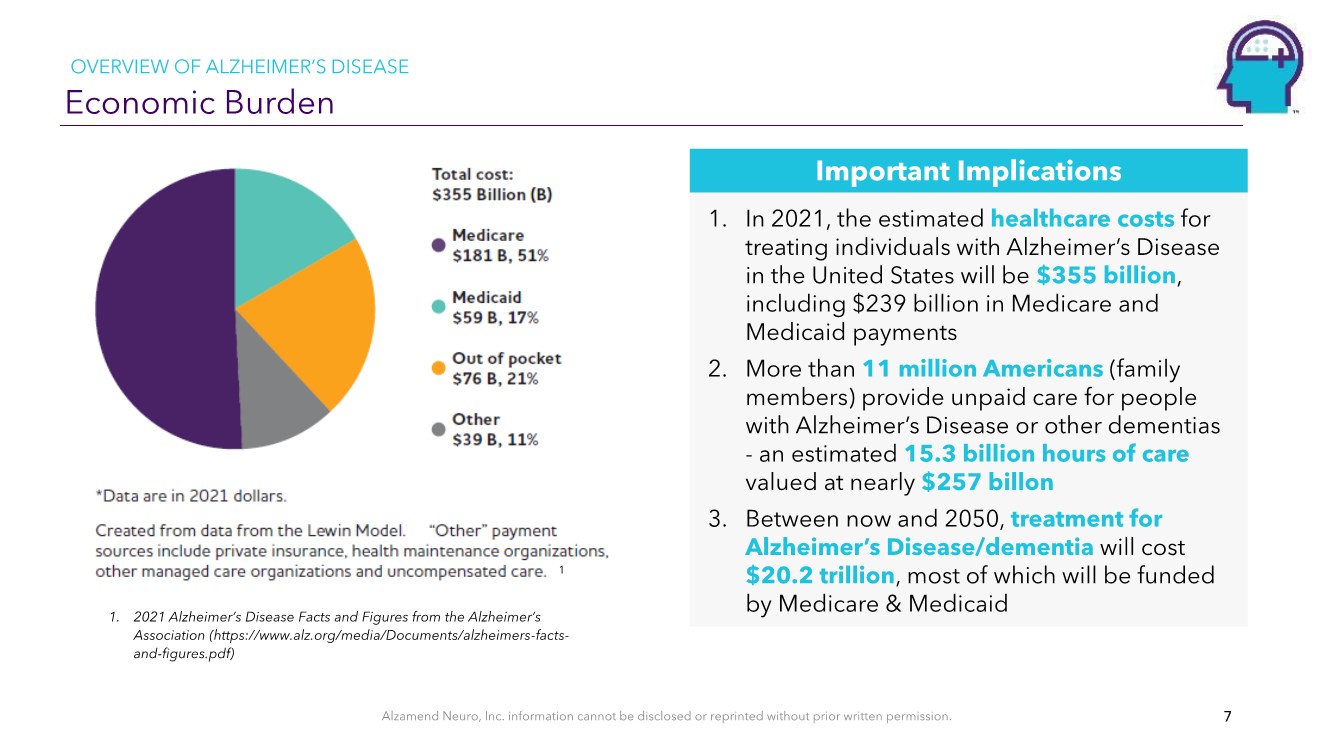
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Economic Burden 1. In 2021, the estimated healthcare costs for treating individuals with Alzheimer’s Disease in the United States will be $355 billion, including $239 billion in Medicare and Medicaid payments 2. More than 11 million Americans (family members) provide unpaid care for people with Alzheimer’s Disease or other dementias - an estimated 15.3 billion hours of care valued at nearly $257 billon 3. Between now and 2050, treatment for Alzheimer’s Disease/dementia will cost $20.2 trillion, most of which will be funded by Medicare & Medicaid Important Implications 7 1. 2021 Alzheimer’s Disease Facts and Figures from the Alzheimer’s Association (https://www.alz.org/media/Documents/alzheimers-facts- and-figures.pdf) OVERVIEW OF ALZHEIMER’S DISEASE 1 |
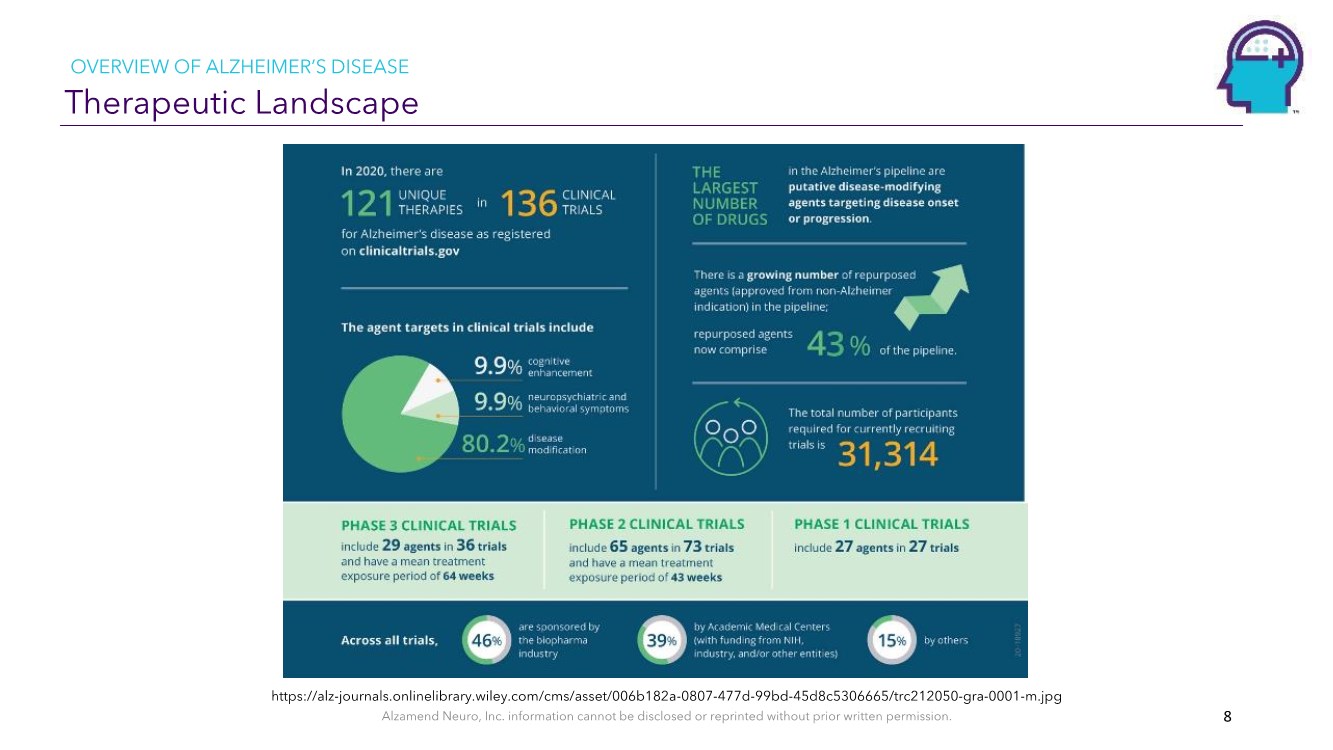
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Therapeutic Landscape 8 https://alz-journals.onlinelibrary.wiley.com/cms/asset/006b182a-0807-477d-99bd-45d8c5306665/trc212050-gra-0001-m.jpg OVERVIEW OF ALZHEIMER’S DISEASE |
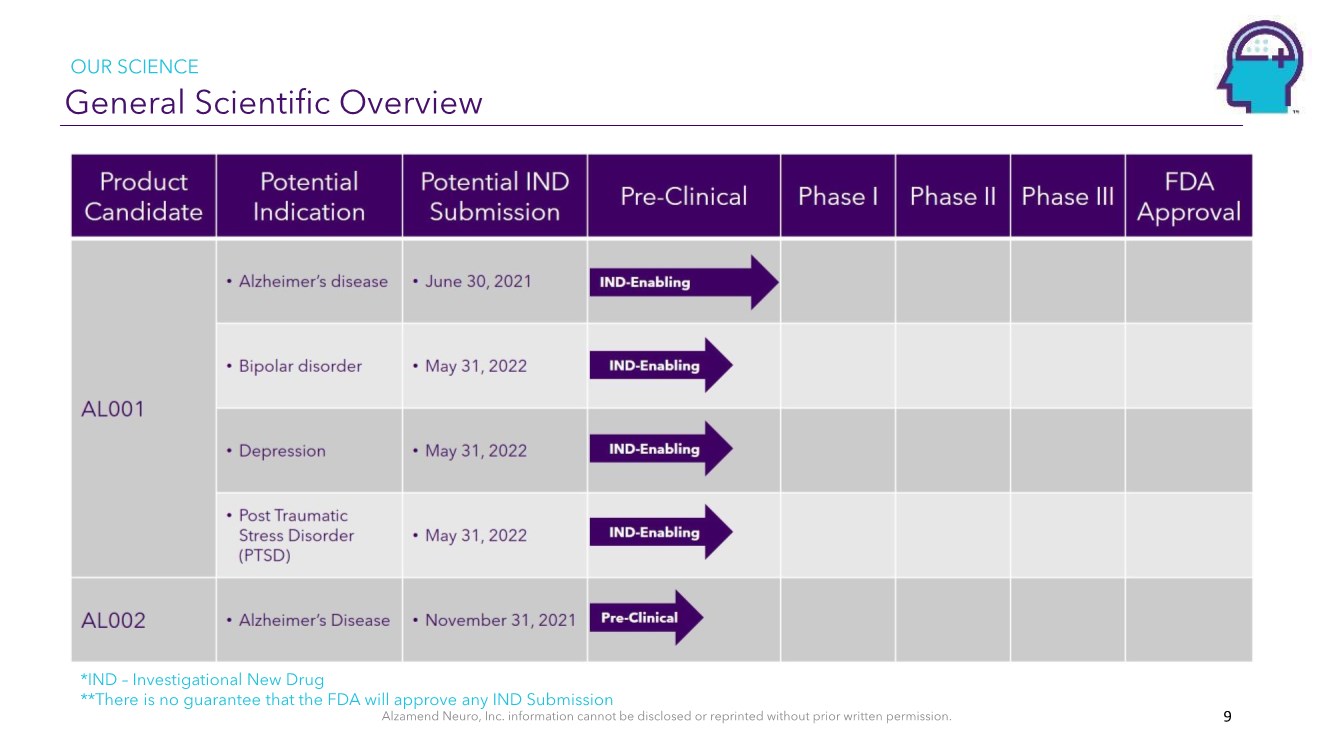
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. General Scientific Overview OUR SCIENCE 9 *IND – Investigational New Drug **There is no guarantee that the FDA will approve any IND Submission |
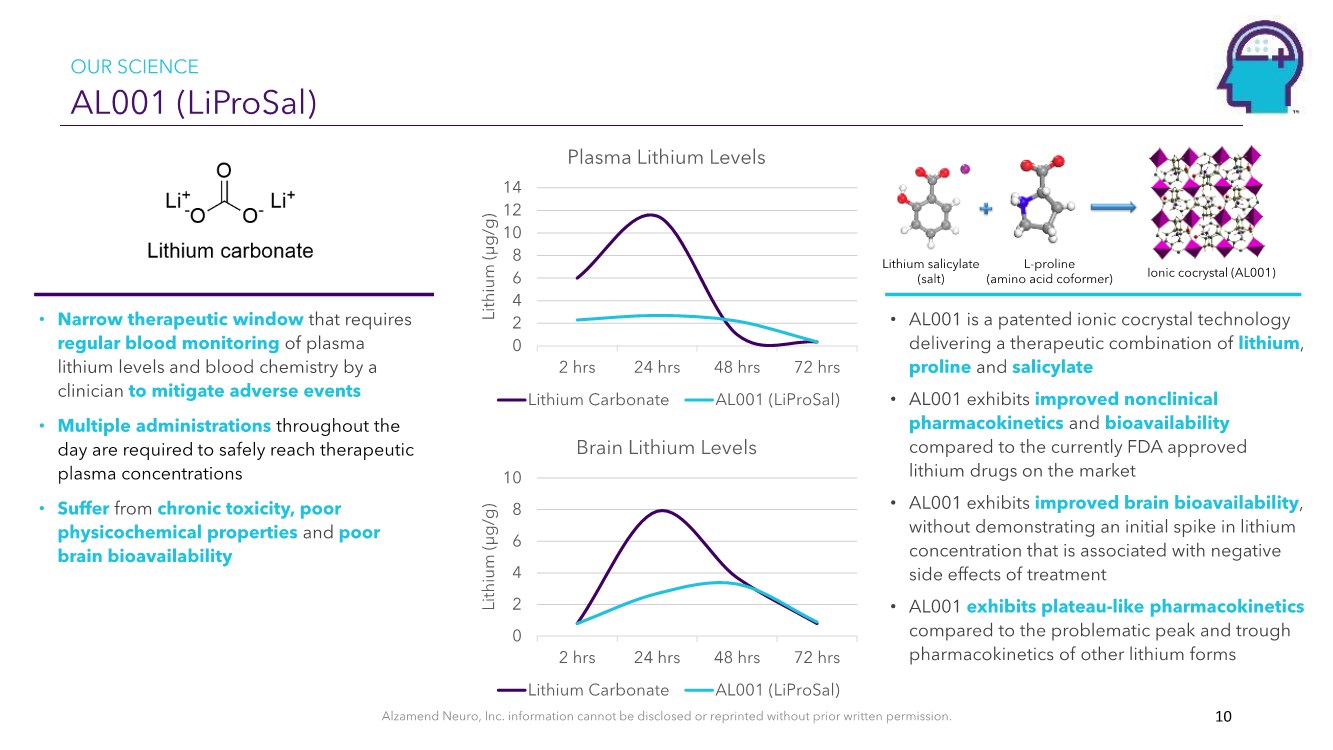
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. AL001 (LiProSal) 10 • AL001 is a patented ionic cocrystal technology delivering a therapeutic combination of lithium, proline and salicylate • AL001 exhibits improved nonclinical pharmacokinetics and bioavailability compared to the currently FDA approved lithium drugs on the market • AL001 exhibits improved brain bioavailability, without demonstrating an initial spike in lithium concentration that is associated with negative side effects of treatment • AL001 exhibits plateau-like pharmacokinetics compared to the problematic peak and trough pharmacokinetics of other lithium forms • Narrow therapeutic window that requires regular blood monitoring of plasma lithium levels and blood chemistry by a clinician to mitigate adverse events • Multiple administrations throughout the day are required to safely reach therapeutic plasma concentrations • Suffer from chronic toxicity, poor physicochemical properties and poor brain bioavailability Ionic cocrystal (AL001) 0 2 4 6 8 10 12 14 2 hrs 24 hrs 48 hrs 72 hrs Lithium (µg/g) Plasma Lithium Levels Lithium Carbonate AL001 (LiProSal) 0 2 4 6 8 10 2 hrs 24 hrs 48 hrs 72 hrs Lithium (µg/g) Brain Lithium Levels Lithium Carbonate AL001 (LiProSal) Lithium salicylate (salt) L-proline (amino acid coformer) OUR SCIENCE |
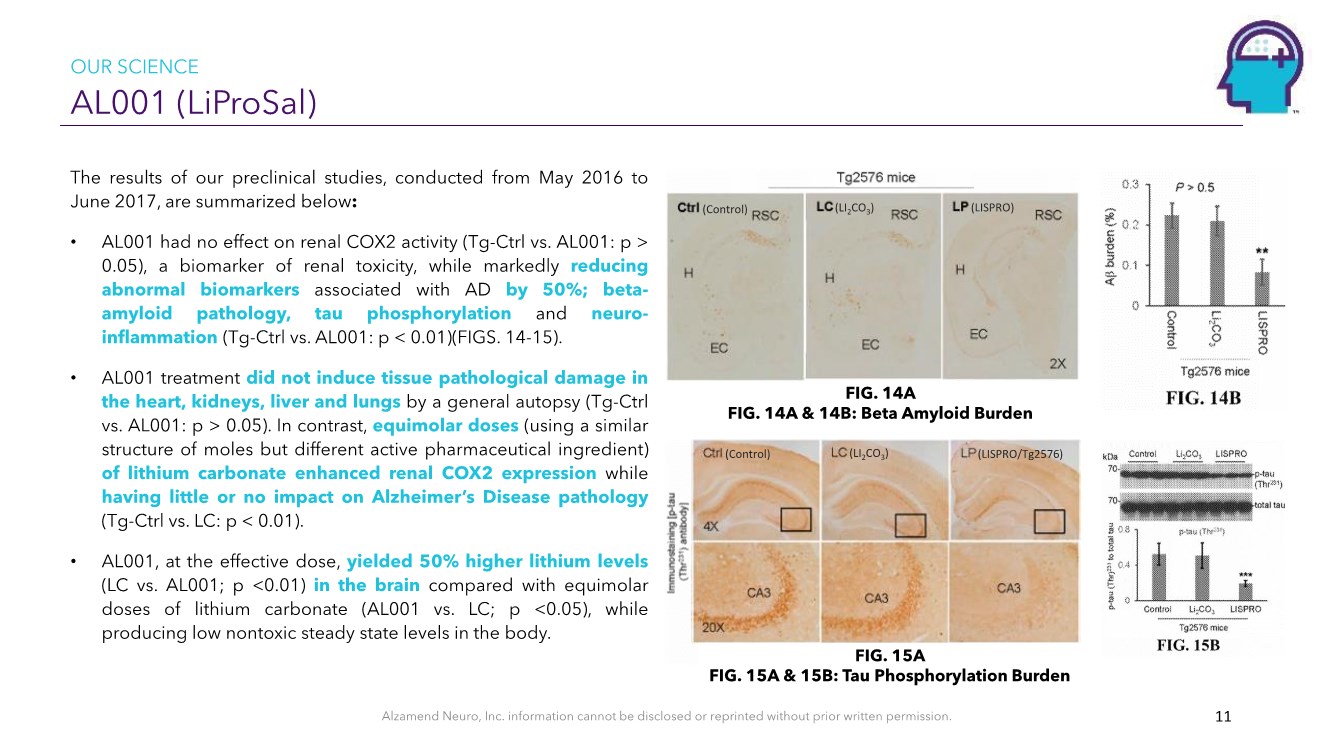
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. AL001 (LiProSal) 11 The results of our preclinical studies, conducted from May 2016 to June 2017, are summarized below: • AL001 had no effect on renal COX2 activity (Tg-Ctrl vs. AL001: p > 0.05), a biomarker of renal toxicity, while markedly reducing abnormal biomarkers associated with AD by 50%; beta- amyloid pathology, tau phosphorylation and neuro- inflammation (Tg-Ctrl vs. AL001: p < 0.01)(FIGS. 14-15). • AL001 treatment did not induce tissue pathological damage in the heart, kidneys, liver and lungs by a general autopsy (Tg-Ctrl vs. AL001: p > 0.05). In contrast, equimolar doses (using a similar structure of moles but different active pharmaceutical ingredient) of lithium carbonate enhanced renal COX2 expression while having little or no impact on Alzheimer’s Disease pathology (Tg-Ctrl vs. LC: p < 0.01). • AL001, at the effective dose, yielded 50% higher lithium levels (LC vs. AL001; p <0.01) in the brain compared with equimolar doses of lithium carbonate (AL001 vs. LC; p <0.05), while producing low nontoxic steady state levels in the body. (LI2CO3)(LISPRO/Tg2576) (Control) (LI2CO3)(Control) (LISPRO) OUR SCIENCE FIG. 14A FIG. 14A & 14B: Beta Amyloid Burden FIG. 15A FIG. 15A & 15B: Tau Phosphorylation Burden |
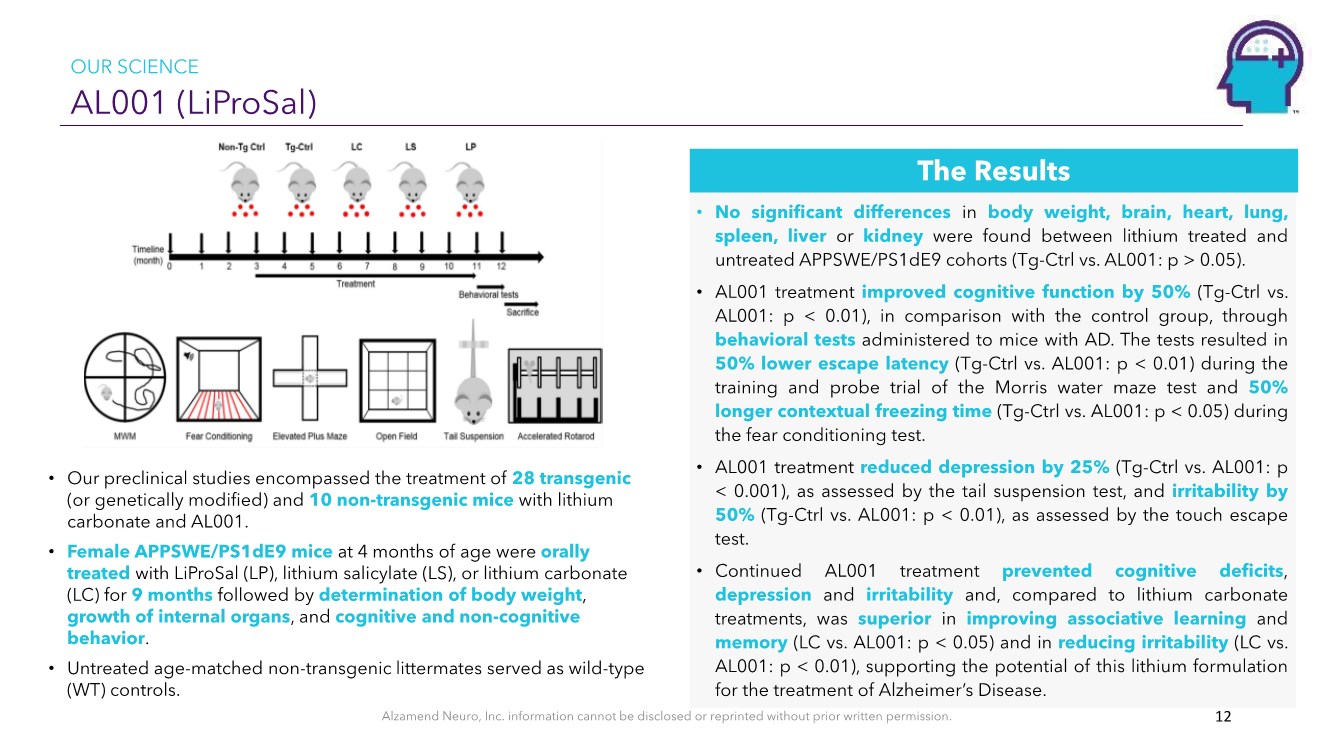
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. AL001 (LiProSal) 12 • Our preclinical studies encompassed the treatment of 28 transgenic (or genetically modified) and 10 non-transgenic mice with lithium carbonate and AL001. • Female APPSWE/PS1dE9 mice at 4 months of age were orally treated with LiProSal (LP), lithium salicylate (LS), or lithium carbonate (LC) for 9 months followed by determination of body weight, growth of internal organs, and cognitive and non-cognitive behavior. • Untreated age-matched non-transgenic littermates served as wild-type (WT) controls. • No significant differences in body weight, brain, heart, lung, spleen, liver or kidney were found between lithium treated and untreated APPSWE/PS1dE9 cohorts (Tg-Ctrl vs. AL001: p > 0.05). • AL001 treatment improved cognitive function by 50%(Tg-Ctrl vs. AL001: p < 0.01), in comparison with the control group, through behavioral tests administered to mice with AD. The tests resulted in 50% lower escape latency (Tg-Ctrl vs. AL001: p < 0.01) during the training and probe trial of the Morris water maze test and 50% longer contextual freezing time (Tg-Ctrl vs. AL001: p < 0.05) during the fear conditioning test. • AL001 treatment reduced depression by 25%(Tg-Ctrl vs. AL001: p < 0.001), as assessed by the tail suspension test, and irritability by 50%(Tg-Ctrl vs. AL001: p < 0.01), as assessed by the touch escape test. • Continued AL001 treatment prevented cognitive deficits, depression and irritability and, compared to lithium carbonate treatments, was superior in improving associative learning and memory (LC vs. AL001: p < 0.05) and in reducing irritability (LC vs. AL001: p < 0.01), supporting the potential of this lithium formulation for the treatment of Alzheimer’s Disease. The Results OUR SCIENCE |
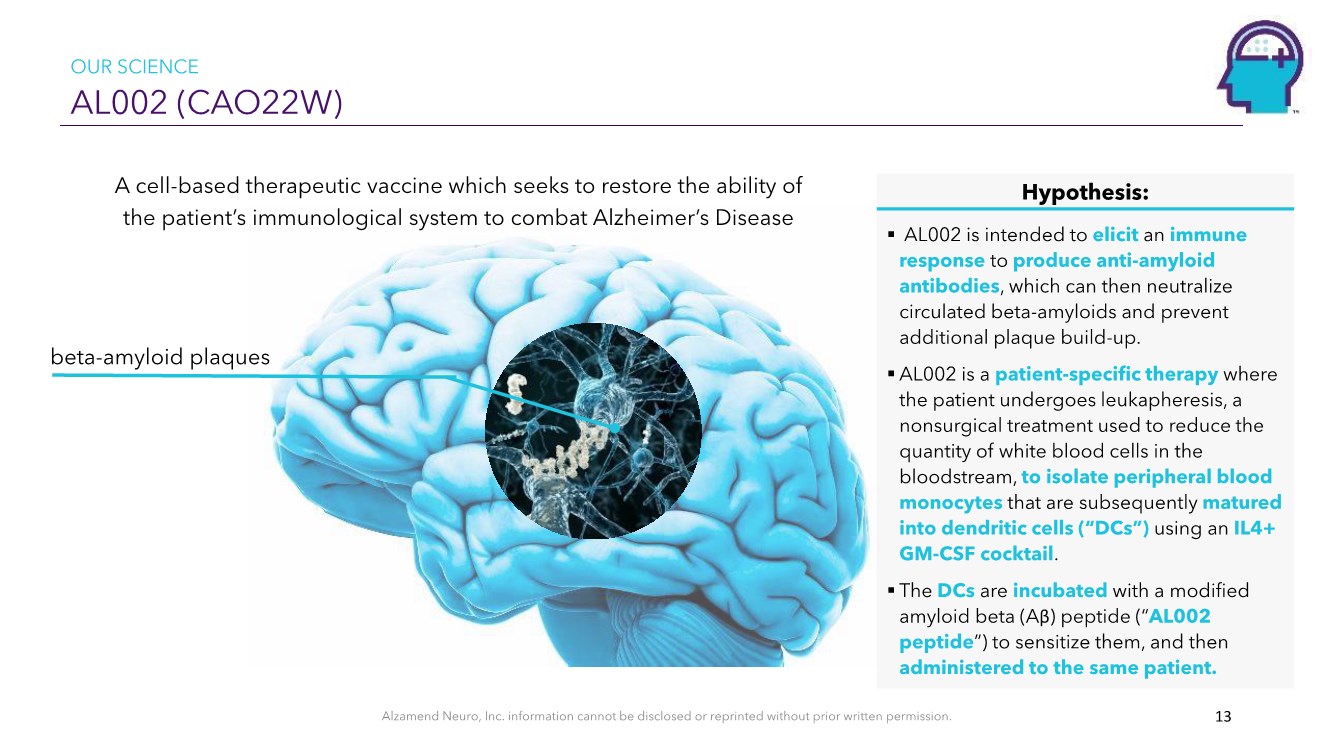
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. AL002 (CAO22W) 13 OUR SCIENCE A cell-based therapeutic vaccine which seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s Disease beta-amyloid plaques Hypothesis: ▪ AL002 is intended to elicit an immune response to produce anti-amyloid antibodies, which can then neutralize circulated beta-amyloids and prevent additional plaque build-up. ▪ AL002 is a patient-specific therapy where the patient undergoes leukapheresis, a nonsurgical treatment used to reduce the quantity of white blood cells in the bloodstream, to isolate peripheral blood monocytes that are subsequently matured into dendritic cells (“DCs”) using an IL4+ GM-CSF cocktail. ▪ The DCs are incubated with a modified amyloid beta (Aβ) peptide (“AL002 peptide”) to sensitize them, and then administered to the same patient. |
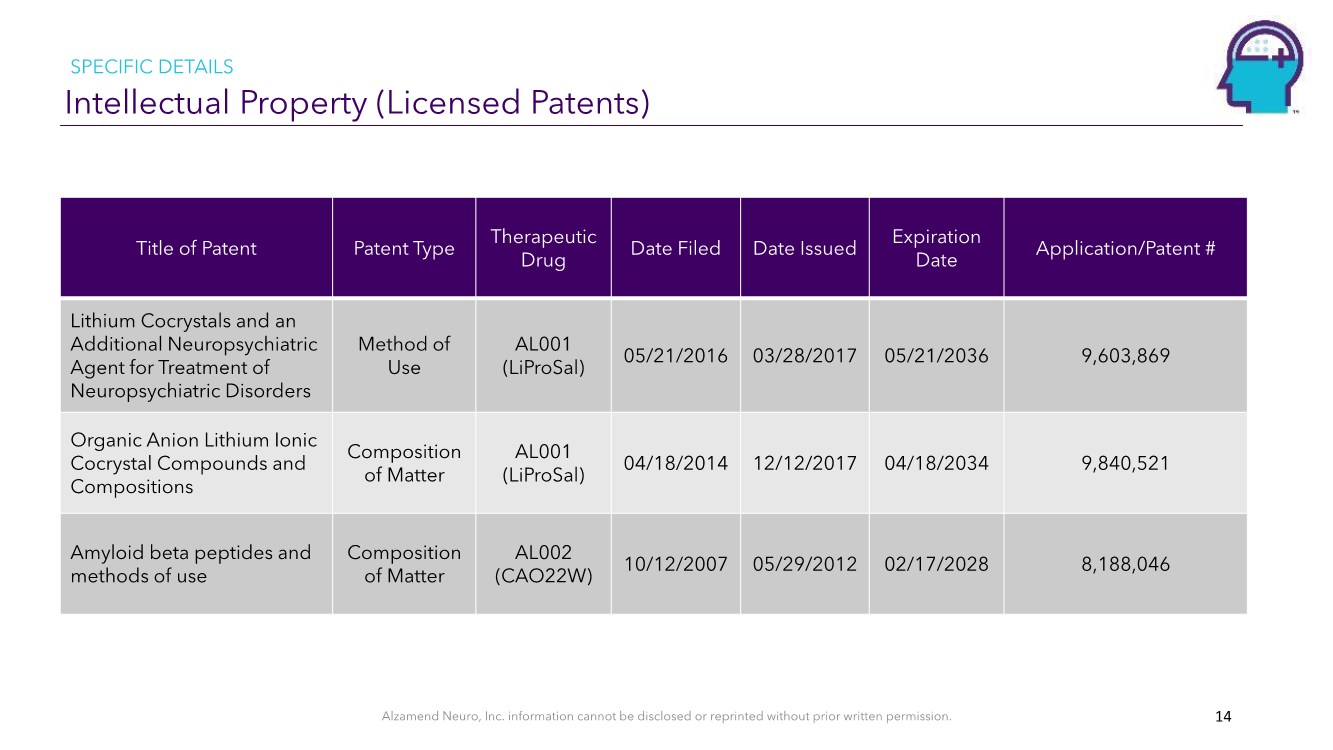
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Intellectual Property (Licensed Patents) SPECIFIC DETAILS 14 Title of Patent Patent Type Therapeutic Drug Date Filed Date Issued Expiration Date Application/Patent # Lithium Cocrystals and an Additional Neuropsychiatric Agent for Treatment of Neuropsychiatric Disorders Method of Use AL001 (LiProSal) 05/21/2016 03/28/2017 05/21/2036 9,603,869 Organic Anion Lithium Ionic Cocrystal Compounds and Compositions Composition of Matter AL001 (LiProSal) 04/18/2014 12/12/2017 04/18/2034 9,840,521 Amyloid beta peptides and methods of use Composition of Matter AL002 (CAO22W) 10/12/2007 05/29/2012 02/17/2028 8,188,046 |
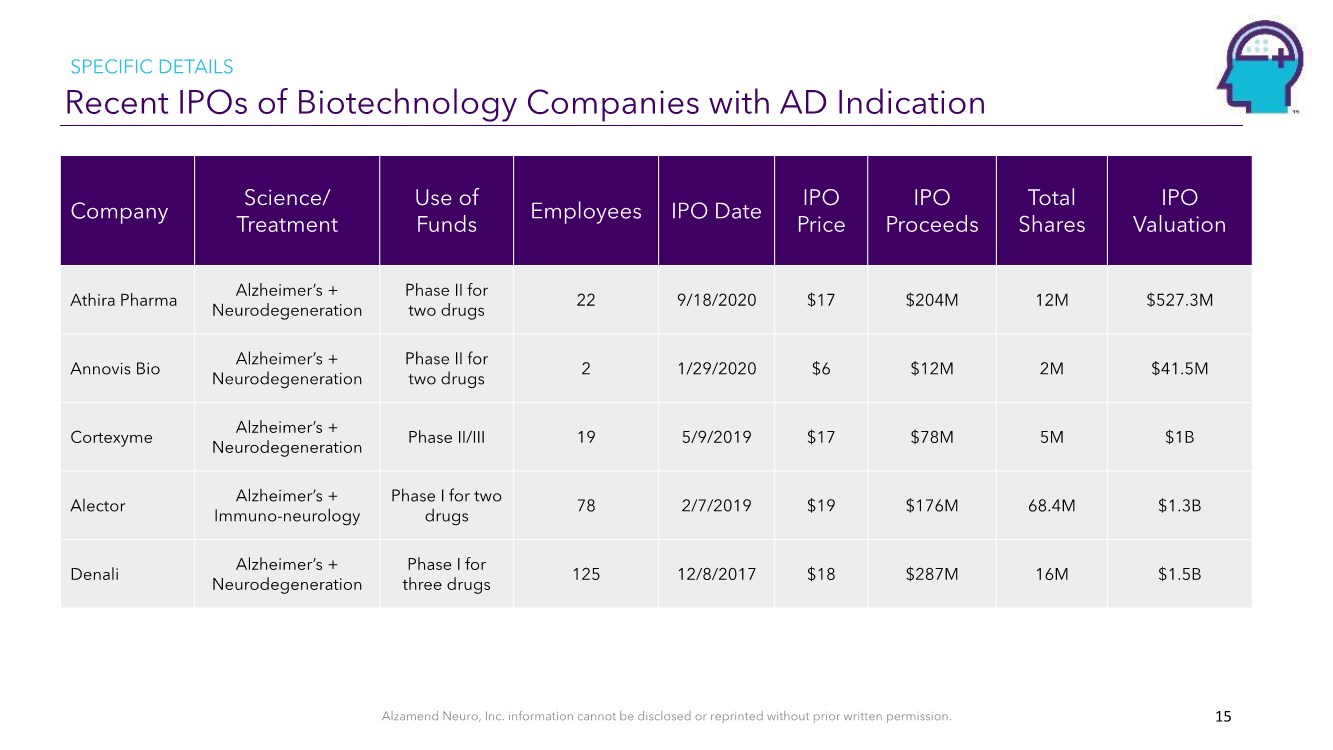
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Recent IPOs of Biotechnology Companies with AD Indication 15 Company Science/ Treatment Use of Funds Employees IPO Date IPO Price IPO Proceeds Total Shares IPO Valuation Athira Pharma Alzheimer’s + Neurodegeneration Phase II for two drugs 22 9/18/2020 $17 $204M 12M $527.3M Annovis Bio Alzheimer’s + Neurodegeneration Phase II for two drugs 2 1/29/2020 $6 $12M 2M $41.5M Cortexyme Alzheimer’s + Neurodegeneration Phase II/III 19 5/9/2019 $17 $78M 5M $1B Alector Alzheimer’s + Immuno-neurology Phase I for two drugs 78 2/7/2019 $19 $176M 68.4M $1.3B Denali Alzheimer’s + Neurodegeneration Phase I for three drugs 125 12/8/2017 $18 $287M 16M $1.5B SPECIFIC DETAILS |
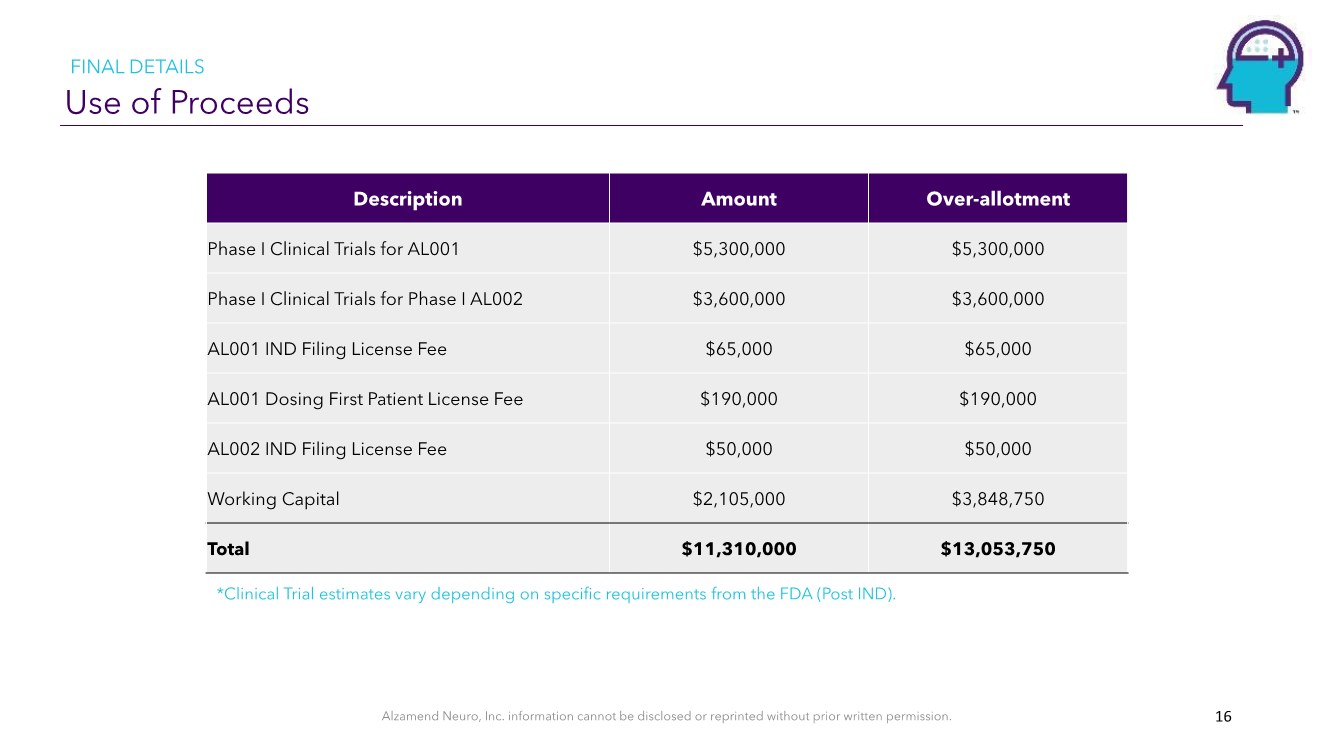
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Use of Proceeds 16 *Clinical Trial estimates vary depending on specific requirements from the FDA (Post IND). Description Amount Over-allotment Phase I Clinical Trials for AL001 $5,300,000 $5,300,000 Phase I Clinical Trials for Phase I AL002 $3,600,000 $3,600,000 AL001 IND Filing License Fee $65,000 $65,000 AL001 Dosing First Patient License Fee $190,000 $190,000 AL002 IND Filing License Fee $50,000 $50,000 Working Capital $2,105,000 $3,848,750 Total $11,310,000 $13,053,750 FINAL DETAILS |
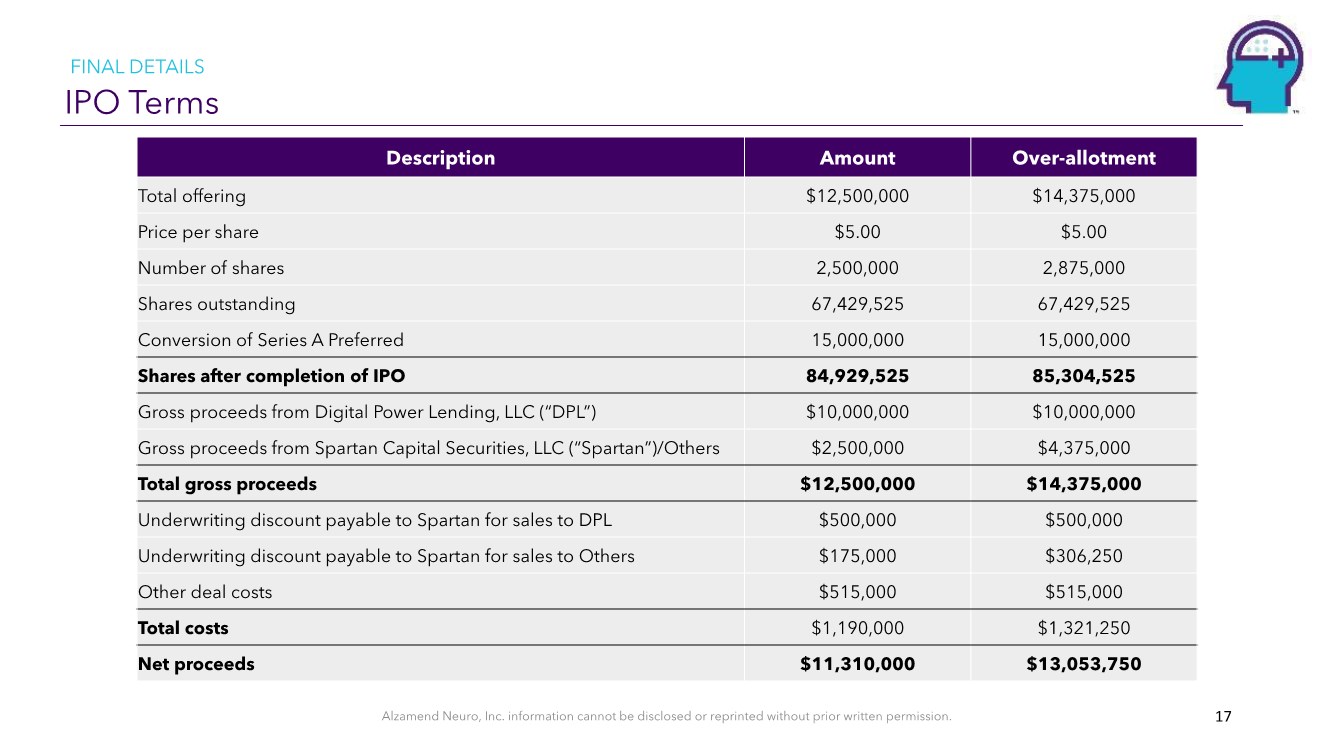
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. IPO Terms 17 Description Amount Over-allotment Total offering $12,500,000 $14,375,000 Price per share $5.00 $5.00 Number of shares 2,500,000 2,875,000 Shares outstanding 67,429,525 67,429,525 Conversion of Series A Preferred 15,000,000 15,000,000 Shares after completion of IPO 84,929,525 85,304,525 Gross proceeds from Digital Power Lending, LLC (“DPL”) $10,000,000 $10,000,000 Gross proceeds from Spartan Capital Securities, LLC (“Spartan”)/Others $2,500,000 $4,375,000 Total gross proceeds $12,500,000 $14,375,000 Underwriting discount payable to Spartan for sales to DPL $500,000 $500,000 Underwriting discount payable to Spartan for sales to Others $175,000 $306,250 Other deal costs $515,000 $515,000 Total costs $1,190,000 $1,321,250 Net proceeds $11,310,000 $13,053,750 FINAL DETAILS |
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend Leadership Team ALZAMEND NEURO 18 Stephan Jackman Chief Executive Officer and Director 20+ years multi-industry experience, specialized in Biotech and Pharmaceutical Lien T. Escalona Chief Financial Officer 25+ years multi-industry experience with an emphasis on accounting and finance, system implementation and SEC reporting Kenneth S. Cragun Senior Vice President of Finance 30+ years SEC reporting, CFO of publicly-traded company on Nasdaq, multi-industry experience, including Biotech and Healthcare Henry Nisser Executive Vice President, General Counsel and Director 20+ years experience, U.S. securities compliance, M&A, equity/debt financings and corporate governance David J. Katzoff Chief Operating Officer 30+ years multi-industry experience, including Healthcare and Technology |
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend Board of Directors 19 Lynne Fahey McGrath Regulatory Affairs and Product Development Consultant 30+ years experience, Biotech and Pharmaceuticals M.P.H./Ph.D., Public Health from UMDNJ – Robert Wood Johnson Medical School Andrew H. Woo M.D., Ph.D. Practicing physician at Santa Monica Neurological Consultants, Assistant Clinical Professor of Neurology at the David Geffen School of Medicine at UCLA and Cedars-Sinai Medical Center 20+ years experience in Psychiatry and Neurology Mark Gustafson C.P.A. Chief Financial Officer of PharmaKure Limited 30+ years multi-industry experience as an active CPA, specialized in Biotech, Energy and Technology Milton “Todd” Ault, III Founder/Chairman Emeritus of Alzamend Executive Chairman of Ault Global Holdings 27+ years Financial Industry experience, seasoned Wall Street CEO & activist investor FINAL DETAILS William B. Horne Chairman of Alzamend Chief Executive Officer of Ault Global Holdings 25+ years Financial Industry experience, prior “Big 4” auditor and healthcare executive Stephan Jackman Chief Executive Officer and Director 20+ years multi-industry experience, specialized in Biotech and Pharmaceutical Henry Nisser Executive Vice President, General Counsel and Director 20+ years experience, U.S. securities compliance, M&A, equity/debt financings and corporate governance Jeffrey Oram Principal at Godby Realtors 25+ years multi-industry experience, Investments, Real Estate and Technology |
| Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend Scientific Advisory Board 20 Eric McDade, DO Associate Director of the Dominantly Inherited Alzheimer Network Trials Unit (“DIAN-TU”), Washington University School of Medicine 76+ Peer-Reviewed Journal Publications Leads a Research Laboratory Continuously Funded by the National Institutes of Health for 10+ Years Thomas M. Wisniewski, MD Director, NYU Langone’s Pearl I. Barlow Center for Memory Evaluation and Treatment 300+ Peer-Reviewed Medical Journal Publications (28 U.S. Patents Issued) Leads a Research Laboratory Continuously Funded by the National Institutes of Health for 30+ Years FINAL DETAILS |
| 21 For more information please contact: Stephan Jackman Chief Executive Officer June 2021 Filed Pursuant to Rule 433 Issuer Free Writing Prospectus Registration Statement File No. 333-255955 |