
Exhibit 99.1

Corporate Overview January 2019 TM

Safe Harbor Disclaimer This presentation contains forward - looking statements. All statements other than statements of historical fact are, or may be de emed to be, forward - looking statements. Such forward - looking statements include statements regarding, among others, (a) our expectations about possible business combinations, (b) our growth strategies, (c) our future financing plans, an d (d) our anticipated needs for working capital. Forward - looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words “may,” “will,” “should,” “expe ct, ” “anticipate,” “approximate,” “estimate,” “believe,” “intend,” “plan,” “budget,” “could,” “forecast,” “might,” “predict,” “shall” or “project,” or the negative of these words or other variations on these words or comparable terminology. Th is information may involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from the future results, performance, or achievements expres sed or implied by any forward - looking statements. These statements may be found in the Annual Report and in the Semiannual Report referred to immediately below. This presentation should be read in conjunction with the audited financial statements and related notes for the fiscal year e nde d April 30, 2017, contained in Alzamend’s™, or the Company’s, Annual Report on Form 1 - K, as well as the condensed financial statements and related notes for the six months ended October 31, 2017, contained in the Company’s Semiannual Repor t o n Form 1 - SA, as filed with the Securities and Exchange Commission on January 29, 2018 and January 30, 2018, respectively. Forward - looking statements are based on our current expectations and assumptions regarding our business, potential target busine sses, the economy and other future conditions. Because forward - looking statements relate to the future, by their nature, they are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward - looking statements as a result of various factors, including, without limitation, changes in local, regional, national or global political, economic, business, competi tiv e, market (supply and demand) and regulatory conditions and the following: ■ Our ability to effectively execute our business plan; ■ Our ability to manage our expansion, growth and operating expenses; ■ Our ability to evaluate and measure our business, prospects and performance metrics; ■ Our ability to compete and succeed in a highly competitive and evolving industry; ■ Our ability to respond and adapt to changes in technology and customer behavior; and ■ Our ability to protect our intellectual property and to develop, maintain and enhance a strong brand. We caution you therefore that you should not rely on any of these forward - looking statements as statements of historical fact or as guarantees or assurances of future performance. All forward - looking statements speak only as of the date of this presentation. We undertake no obligation to update any forward - looking statements or other information contained herein. Information regarding market and industry statistics contained in this presentation is included based on information availabl e t o us that we believe is accurate. It is generally based on academic and other publications that are not produced for purposes of securities offerings or economic analysis. Forecasts and other forward - looking information obtained from these sourc es are subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. Except as required by U.S. federal securities laws, we h ave no obligation to update forward - looking information to reflect actual results or changes in assumptions or other factors that could affect those statements. January 2019 Alzamend ™ 2 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.

Mission & Motto “To help the Alzheimer’s community through the support of research and the commercialization of preventions, treatments and cures of this devastating disease.” January 2019 Join Alzamend™ in “Making Alzheimer’s Just a Memory ”! ™ Alzamend ™ 3 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.

January 2019 Alzamend Neuro™ was founded in 2016 by Milton “Todd” Ault, III because of a lifelong goal to find a treatment/cure for Alzheimer’s and other neurodegenerative diseases that have plagued his family for generations. His unwavering passion and commitment led him to the University of South Florida (USF) Health Byrd Alzheimer’s Center and Research Institute, one the top 20 institutions in the nation for patented research and their portfolio of proprietary solutions. The Company has licensed two patented therapeutic compounds indicated for the treatment and prevention of Alzheimer’s disease. Our Story Alzamend ™ 4 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc. G1

Our Team January 2019 Alzamend ™ Stephan Jackman Chief Executive Officer 20+ years multi - industry experience, specialized in Biotech and Pharmaceutical Kenneth S. Cragun Chief Financial Officer/Treasurer 30+ Years multi - industry experience, including Biotech and Healthcare Gary Gottlieb Director, Bus. Dev. & Administration 35+ Years multi - industry experience, including Biotech and Healthcare Milton “Todd” Ault, III Founder/Executive Chairman 27+ years Financial Industry experience, seasoned Wall Street CEO & activist investor Philip E. Mansour Board of Directors 25+ years multi - industry experience, seasoned executive, manager & coach William B. Horne Board of Directors 25+ years Financial Industry experience, prior “Big 4” auditor & healthcare executive 5 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
January 2019 We intend to seek and acquire early - stage, proprietary, innovative science residing on research benchtop shelves awaiting commercialization with the patent life ticking away. There is a 20+ year backlog of scientific discoveries that may be useful in human medicine. Alzamend™ anticipates finding these discoveries and fast - track developing them to fulfill our mission; to bring treatments/cures to those suffering. Alzamend™ intends to secure funding by leveraging traditional channels, directed at accredited and institutional investors, and new financial regulations which permit direct marketing to the public. This approach will increase the speed, volume and breadth of capital raised while concurrently minimizing overhead costs. Our Approach Alzamend ™ 6 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.

Alzheimer’s Disease - The sixth leading cause of death in the United States Alzheimer’s disease (“AD”) is an irreversible, progressive brain disorder that slowly destroys memory and thinking skills, and eventually the ability to carry out the simplest tasks. In most people with Alzheimer’s, symptoms first appear in their early to mid - 60’s. Estimates vary, but experts suggest that more than 5.7 million Americans may have AD, considered by many as “the most feared” disease. Alzheimer’s has no current cure , but four treatments for symptoms are available today while research continues. Alzamend ™ January 2019 7 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
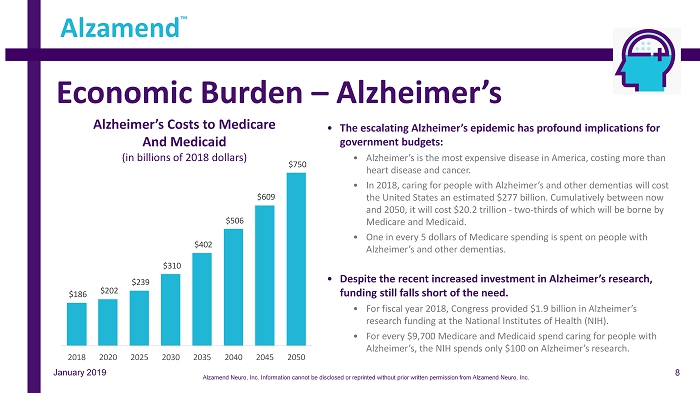
Economic Burden – Alzheimer’s • The escalating Alzheimer’s epidemic has profound implications for government budgets: • Alzheimer’s is the most expensive disease in America, costing more than heart disease and cancer. • In 2018, caring for people with Alzheimer’s and other dementias will cost the United States an estimated $277 billion. Cumulatively between now and 2050, it will cost $20.2 trillion - two - thirds of which will be borne by Medicare and Medicaid. • One in every 5 dollars of Medicare spending is spent on people with Alzheimer’s and other dementias. • Despite the recent increased investment in Alzheimer’s research, funding still falls short of the need. • For fiscal year 2018, Congress provided $1.9 billion in Alzheimer’s research funding at the National Institutes of Health (NIH). • For every $9,700 Medicare and Medicaid spend caring for people with Alzheimer’s, the NIH spends only $100 on Alzheimer’s research. Alzamend ™ January 2019 $186 $202 $239 $310 $402 $506 $609 $750 2018 2020 2025 2030 2035 2040 2045 2050 Alzheimer’s Costs to Medicare And Medicaid (in billions of 2018 dollars) 8 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
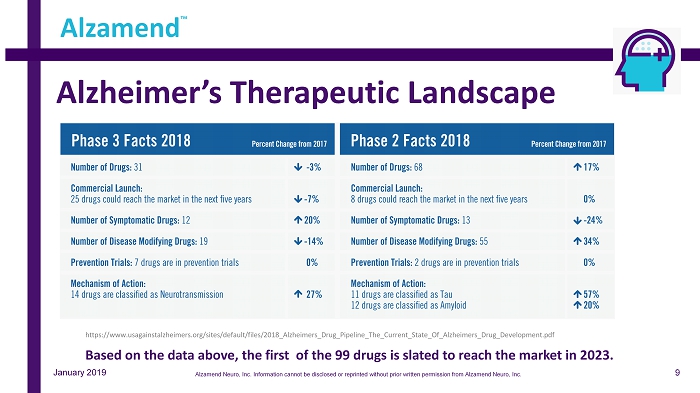
Alzheimer’s Therapeutic Landscape Alzamend ™ https://www.usagainstalzheimers.org/sites/default/files/2018_Alzheimers_Drug_Pipeline_The_Current_State_Of_Alzheimers_Drug_De vel opment.pdf January 2019 Based on the data above, the first of the 99 drugs is slated to reach the market in 2023. 9 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
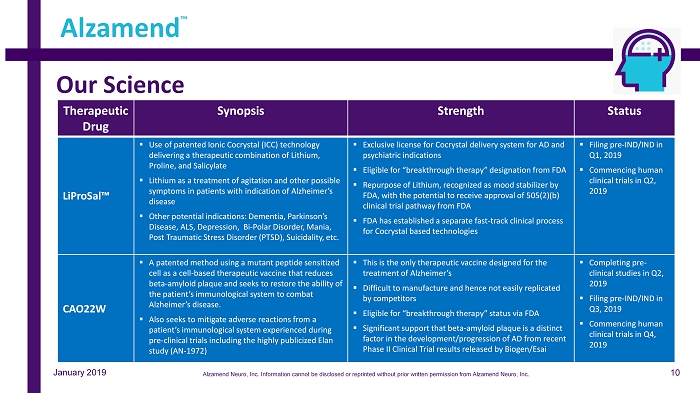
Our Science Alzamend ™ January 2019 Therapeutic Drug Synopsis Strength Status LiProSal™ ▪ Use of patented Ionic Cocrystal (ICC) technology delivering a therapeutic combination of Lithium, Proline, and Salicylate ▪ Lithium as a treatment of agitation and other possible symptoms in patients with indication of Alzheimer’s disease ▪ Other potential indications: Dementia, Parkinson’s Disease, ALS, Depression, Bi - Polar Disorder, Mania, Post Traumatic Stress Disorder (PTSD), Suicidality, etc. ▪ Exclusive license for Cocrystal delivery system for AD and psychiatric indications ▪ Eligible for “breakthrough therapy” designation from FDA ▪ Repurpose of Lithium, recognized as mood stabilizer by FDA, with the potential to receive approval of 505(2)(b) clinical trial pathway from FDA ▪ FDA has established a separate fast - track clinical process for Cocrystal based technologies ▪ Filing pre - IND/IND in Q1, 2019 ▪ Commencing human clinical trials in Q2, 2019 CAO22W ▪ A patented method using a mutant peptide sensitized cell as a cell - based therapeutic vaccine that reduces beta - amyloid plaque and seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s disease. ▪ Also seeks to mitigate adverse reactions from a patient’s immunological system experienced during pre - clinical trials including the highly publicized Elan study (AN - 1972) ▪ This is the only therapeutic vaccine designed for the treatment of Alzheimer’s ▪ Difficult to manufacture and hence not easily replicated by competitors ▪ Eligible for “breakthrough therapy” status via FDA ▪ Significant support that beta - amyloid plaque is a distinct factor in the development/progression of AD from recent Phase II Clinical Trial results released by Biogen/Esai ▪ Completing pre - clinical studies in Q2, 2019 ▪ Filing pre - IND/IND in Q3, 2019 ▪ Commencing human clinical trials in Q4, 2019 10 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
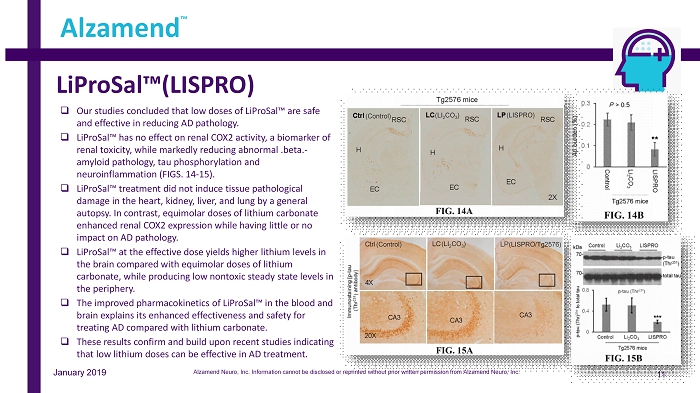
LiProSal™(LISPRO) 11 Alzamend ™ January 2019 □ Our studies concluded that low doses of LiProSal™ are safe and effective in reducing AD pathology. □ LiProSal™ has no effect on renal COX2 activity, a biomarker of renal toxicity, while markedly reducing abnormal .beta. - amyloid pathology, tau phosphorylation and neuroinflammation (FIGS. 14 - 15). □ LiProSal™ treatment did not induce tissue pathological damage in the heart, kidney, liver, and lung by a general autopsy. In contrast, equimolar doses of lithium carbonate enhanced renal COX2 expression while having little or no impact on AD pathology. □ LiProSal™ at the effective dose yields higher lithium levels in the brain compared with equimolar doses of lithium carbonate, while producing low nontoxic steady state levels in the periphery. □ The improved pharmacokinetics of LiProSal™ in the blood and brain explains its enhanced effectiveness and safety for treating AD compared with lithium carbonate. □ These results confirm and build upon recent studies indicating that low lithium doses can be effective in AD treatment. (LI 2 CO 3 ) (LISPRO/Tg2576) (Control) (LI 2 CO 3 ) (Control) (LISPRO) Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
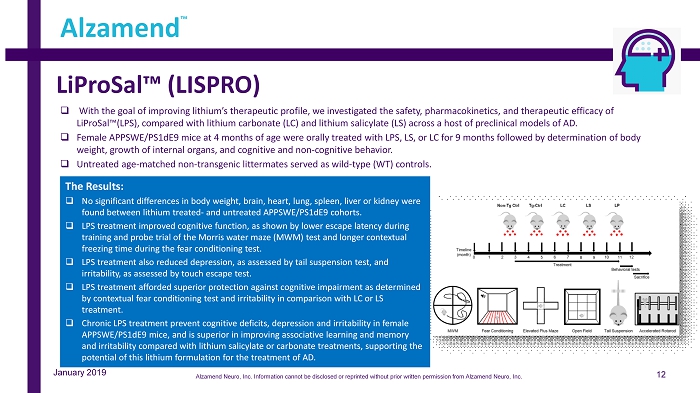
LiProSal™ (LISPRO) 12 Alzamend ™ January 2019 □ With the goal of improving lithium’s therapeutic profile, we investigated the safety, pharmacokinetics, and therapeutic effic ac y of LiProSal™(LPS), compared with lithium carbonate (LC) and lithium salicylate (LS) across a host of preclinical models of AD. □ Female APPSWE/PS1dE9 mice at 4 months of age were orally treated with LPS, LS, or LC for 9 months followed by determination o f b ody weight, growth of internal organs, and cognitive and non - cognitive behavior. □ Untreated age - matched non - transgenic littermates served as wild - type (WT) controls. The Results: □ No significant differences in body weight, brain, heart, lung, spleen, liver or kidney were found between lithium treated - and untreated APPSWE/PS1dE9 cohorts. □ LPS treatment improved cognitive function, as shown by lower escape latency during training and probe trial of the Morris water maze (MWM) test and longer contextual freezing time during the fear conditioning test. □ LPS treatment also reduced depression, as assessed by tail suspension test, and irritability, as assessed by touch escape test. □ LPS treatment afforded superior protection against cognitive impairment as determined by contextual fear conditioning test and irritability in comparison with LC or LS treatment. □ Chronic LPS treatment prevent cognitive deficits, depression and irritability in female APPSWE/PS1dE9 mice, and is superior in improving associative learning and memory and irritability compared with lithium salicylate or carbonate treatments, supporting the potential of this lithium formulation for the treatment of AD. Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
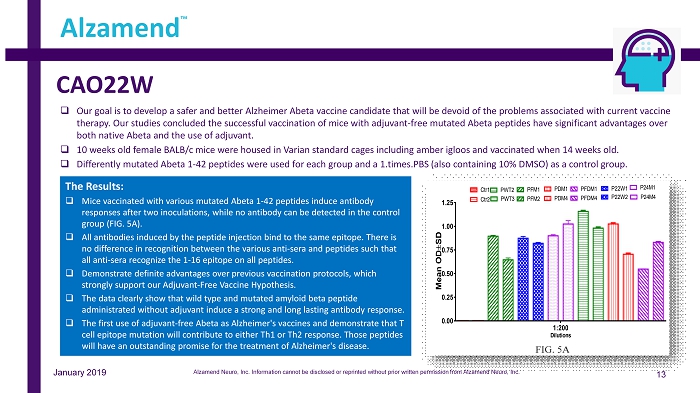
CAO22W 13 Alzamend ™ January 2019 □ Our goal is to develop a safer and better Alzheimer Abeta vaccine candidate that will be devoid of the problems associated wi th current vaccine therapy. Our studies concluded the successful vaccination of mice with adjuvant - free mutated Abeta peptides have significant advantages over both native Abeta and the use of adjuvant. □ 10 weeks old female BALB/c mice were housed in Varian standard cages including amber igloos and vaccinated when 14 weeks old. □ Differently mutated Abeta 1 - 42 peptides were used for each group and a 1.times.PBS (also containing 10% DMSO) as a control group. The Results: □ Mice vaccinated with various mutated Abeta 1 - 42 peptides induce antibody responses after two inoculations, while no antibody can be detected in the control group (FIG. 5A). □ All antibodies induced by the peptide injection bind to the same epitope. There is no difference in recognition between the various anti - sera and peptides such that all anti - sera recognize the 1 - 16 epitope on all peptides. □ Demonstrate definite advantages over previous vaccination protocols, which strongly support our Adjuvant - Free Vaccine Hypothesis. □ The data clearly show that wild type and mutated amyloid beta peptide administrated without adjuvant induce a strong and long lasting antibody response. □ The first use of adjuvant - free Abeta as Alzheimer's vaccines and demonstrate that T cell epitope mutation will contribute to either Th1 or Th2 response. Those peptides will have an outstanding promise for the treatment of Alzheimer's disease. Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
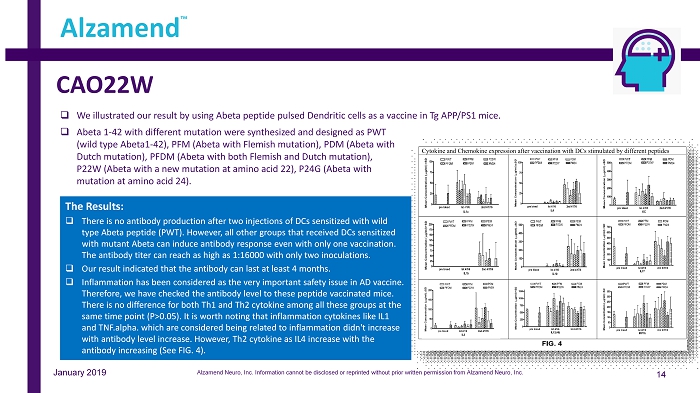
CAO22W Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc. 14 Alzamend ™ January 2019 □ We illustrated our result by using Abeta peptide pulsed Dendritic cells as a vaccine in Tg APP/PS1 mice. The Results: □ There is no antibody production after two injections of DCs sensitized with wild type Abeta peptide (PWT). However, all other groups that received DCs sensitized with mutant Abeta can induce antibody response even with only one vaccination. The antibody titer can reach as high as 1:16000 with only two inoculations. □ Our result indicated that the antibody can last at least 4 months. □ Inflammation has been considered as the very important safety issue in AD vaccine. Therefore, we have checked the antibody level to these peptide vaccinated mice. There is no difference for both Th1 and Th2 cytokine among all these groups at the same time point (P>0.05). It is worth noting that inflammation cytokines like IL1 and TNF.alpha. which are considered being related to inflammation didn't increase with antibody level increase. However, Th2 cytokine as IL4 increase with the antibody increasing (See FIG. 4). □ Abeta 1 - 42 with different mutation were synthesized and designed as PWT (wild type Abeta1 - 42), PFM (Abeta with Flemish mutation), PDM (Abeta with Dutch mutation), PFDM (Abeta with both Flemish and Dutch mutation), P22W (Abeta with a new mutation at amino acid 22), P24G (Abeta with mutation at amino acid 24).
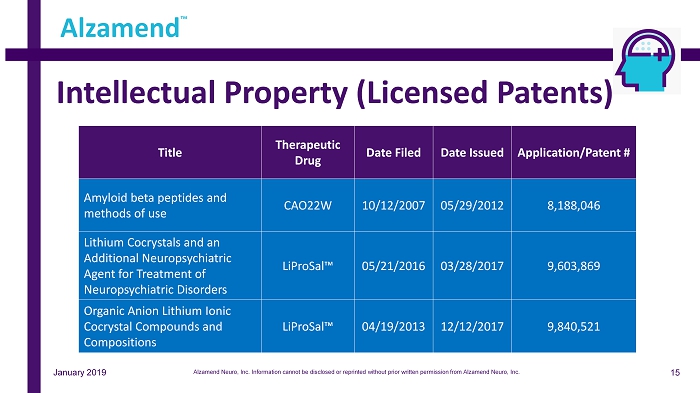
Intellectual Property (Licensed Patents) Alzamend ™ Title Therapeutic Drug Date Filed Date Issued Application/Patent # Amyloid beta peptides and methods of use CAO22W 10/12/2007 05/29/2012 8,188,046 Lithium Cocrystals and an Additional Neuropsychiatric Agent for Treatment of Neuropsychiatric Disorders LiProSal™ 05/21/2016 03/28/2017 9,603,869 Organic Anion Lithium Ionic Cocrystal Compounds and Compositions LiProSal™ 04/19/2013 12/12/2017 9,840,521 January 2019 15 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
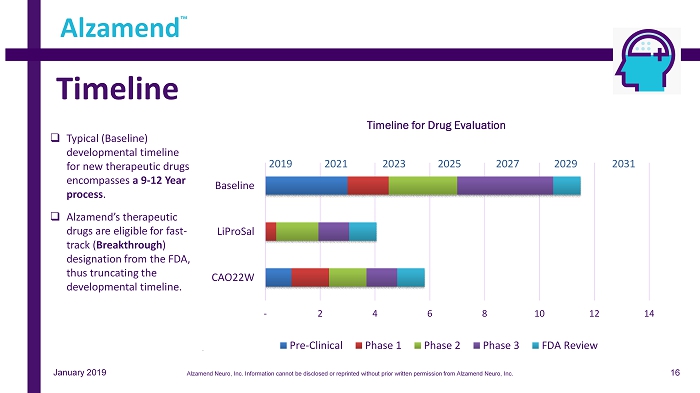
January 2019 Timeline Alzamend ™ - 2 4 6 8 10 12 14 CAO22W LiProSal Baseline Timeline for Drug Evaluation Pre-Clinical Phase 1 Phase 2 Phase 3 FDA Review 2019 20 21 2023 2025 2027 2029 2031 □ Typical (Baseline) developmental timeline for new therapeutic drugs encompasses a 9 - 12 Year process . □ Alzamend’s therapeutic drugs are eligible for fast - track ( Breakthrough ) designation from the FDA, thus truncating the developmental timeline. 16 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
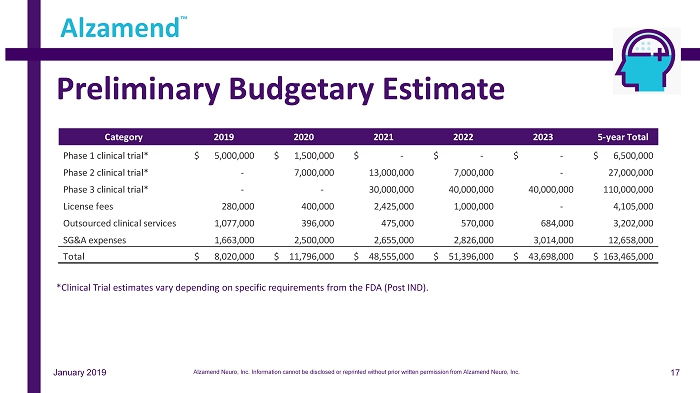
Preliminary Budgetary Estimate Alzamend ™ *Clinical Trial estimates vary depending on specific requirements from the FDA (Post IND). Category 2019 2020 2021 2022 2023 5-year Total Phase 1 clinical trial* 5,000,000$ 1,500,000$ -$ -$ -$ 6,500,000$ Phase 2 clinical trial* - 7,000,000 13,000,000 7,000,000 - 27,000,000 Phase 3 clinical trial* - - 30,000,000 40,000,000 40,000,000 110,000,000 License fees 280,000 400,000 2,425,000 1,000,000 - 4,105,000 Outsourced clinical services 1,077,000 396,000 475,000 570,000 684,000 3,202,000 SG&A expenses 1,663,000 2,500,000 2,655,000 2,826,000 3,014,000 12,658,000 Total 8,020,000$ 11,796,000$ 48,555,000$ 51,396,000$ 43,698,000$ 163,465,000$ 17 January 2019 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
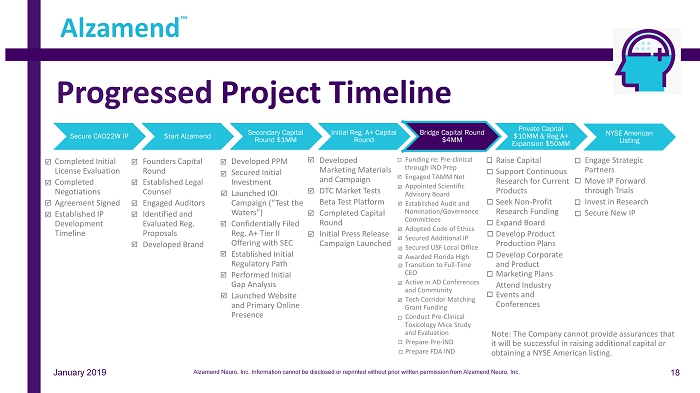
Secure CAO22W IP • Completed Initial License Evaluation • Completed Negotiations • Agreement Signed • Established IP Development Timeline Start Alzamend • Founders Capital Round • Established Legal Counsel • Engaged Auditors • Identified and Evaluated Reg. Proposals • Developed Brand Secondary Capital Round $1MM • Developed PPM • Secured Initial Investment • Launched IOI Campaign (“Test the Waters”) • Confidentially Filed Reg. A+ Tier II Offering with SEC • Established Initial Regulatory Path • Performed Initial Gap Analysis • Launched Website and Primary Online Presence Initial Reg. A+ Capital Round • Developed Marketing Materials and Campaign • DTC Market Tests • Beta Test Platform • Completed Capital Round • Initial Press Release Campaign Launched Bridge Capital Round $4MM • Funding re: Pre - clinical through IND Prep • Engaged TAMM Net • Appointed Scientific Advisory Board • Established Audit and Nomination/Governance Committees • Adopted Code of Ethics • Secured Additional IP • Secured USF Local Office • Awarded Florida High Transition to Full - Time CEO • Active in AD Conferences and Community • Tech Corridor Matching Grant Funding • Conduct Pre - Clinical Toxicology Mice Study and Evaluation • Prepare Pre - IND • Prepare FDA IND Private Capital $10MM & Reg A+ Expansion $50MM • Raise Capital • Support Continuous Research for Current Products • Seek Non - Profit Research Funding • Expand Board • Develop Product Production Plans • Develop Corporate and Product Marketing Plans • Attend Industry Events and Conferences NYSE American Listing • Engage Strategic Partners • Move IP Forward through Trials • Invest in Research • Secure New IP Progressed Project Timeline Alzamend ™ January 2019 18 Note: The Company cannot provide assurances that it will be successful in raising additional capital or obtaining a NYSE American listing. Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
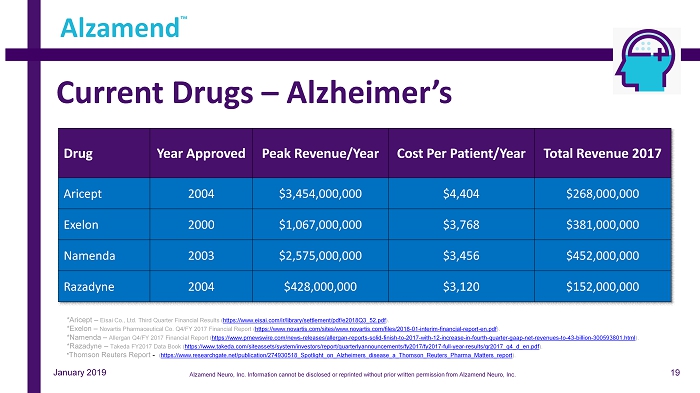
Current Drugs – Alzheimer’s Drug Year Approved Peak Revenue /Year Cost Per Patient/Year Total Revenue 2017 Aricept 2004 $3,454,000,000 $4,404 $268,000,000 Exelon 2000 $1,067,000,000 $3,768 $381,000,000 Namenda 2003 $2,575,000,000 $3,456 $452,000,000 Razadyne 2004 $428,000,000 $3,120 $152,000,000 Alzamend ™ *Aricept – Eisai Co., Ltd. Third Quarter Financial Results ( https://www.eisai.com/ir/library/settlement/pdf/e2018Q3_52.pdf ). *Exelon – Novartis Pharmaceutical Co. Q4/FY 2017 Financial Report ( https://www.novartis.com/sites/www.novartis.com/files/2018 - 01 - interim - financial - report - en.pdf ). *Namenda – Allergan Q4/FY 2017 Financial Report ( https://www.prnewswire.com/news - releases/allergan - reports - solid - finish - to - 2017 - with - 12 - increase - in - fourth - quarter - gaap - net - reven ues - to - 43 - billion - 300593801.html ). *Razadyne – Takeda FY2017 Data Book ( https://www.takeda.com/siteassets/system/investors/report/quarterlyannouncements/fy2017/fy2017 - full - year - results/qr2017_q4_d_en. pdf ). * Thomson Reuters Report - ( https://www.researchgate.net/publication/274930518_Spotlight_on_Alzheimers_disease_a_Thomson_Reuters_Pharma_Matters_report ). January 2019 19 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
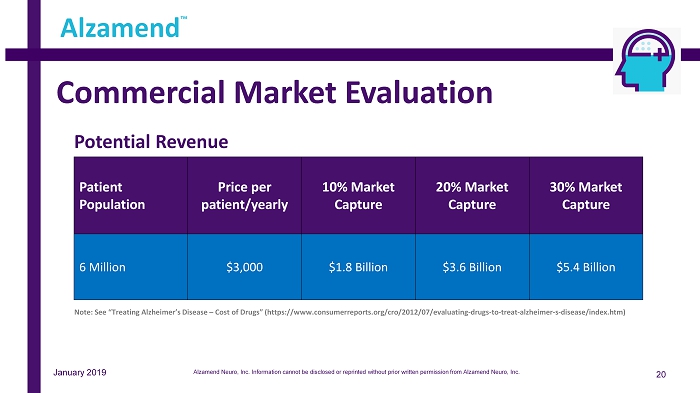
Commercial Market Evaluation 20 Alzamend ™ Note: See “Treating Alzheimer’s Disease – Cost of Drugs” (https://www.consumerreports.org/cro/2012/07/evaluating - drugs - to - treat - alzheimer - s - disease/index.htm) Patient Population Price per patient/yearly 10% Market Capture 20% Market Capture 30% Market Capture 6 Million $3,000 $1.8 Billion $3.6 Billion $5.4 Billion January 2019 Potential Revenue Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
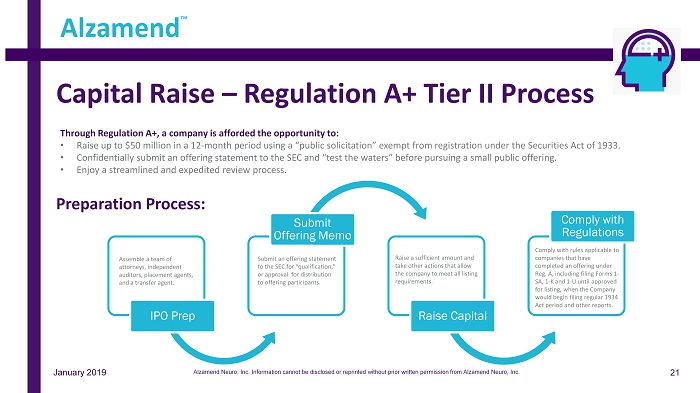
IPO Prep Submit Offering Memo Raise Capital Comply with Regulations Capital Raise – Regulation A+ Tier II Process Assemble a team of attorneys, independent auditors, placement agents, and a transfer agent. Submit an offering statement to the SEC for “qualification,” or approval for distribution to offering participants. Comply with rules applicable to companies that have completed an offering under Reg. A, including filing Forms 1 - SA, 1 - K and 1 - U until approved for listing, when the Company would begin filing regular 1934 Act period and other reports. Raise a sufficient amount and take other actions that allow the company to meet all listing requirements. Through Regulation A+, a company is afforded the opportunity to: • Raise up to $50 million in a 12 - month period using a “public solicitation” exempt from registration under the Securities Act of 1933. • Confidentially submit an offering statement to the SEC and “test the waters” before pursuing a small public offering. • Enjoy a streamlined and expedited review process. Preparation Process: Alzamend ™ January 2019 21 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.
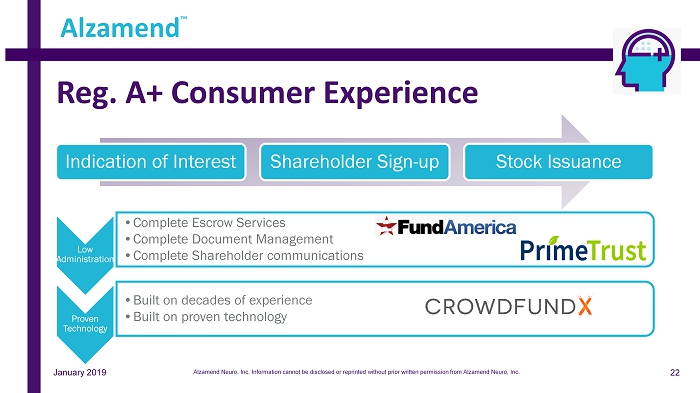
Reg. A+ Consumer Experience Low Administration • Complete Escrow Services • Complete Document Management • Complete Shareholder communications Proven Technology • Built on decades of experience • Built on proven technology Indication of Interest Shareholder Sign - up Stock Issuance Alzamend ™ January 2019 22 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.

Fighting Together to “Make Alzheimer’s Just a Memory”!™ Strategic Partners Alzamend ™ January 2019 23 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc.

TM