
Exhibit 99.1

Corporate Overview February 2019 TM

Safe Harbor Disclaimer This presentation contains forward - looking statements. All statements other than statements of historical fact are, or may be de emed to be, forward - looking statements. Such forward - looking statements include statements regarding, among others, (a) our expectations about possible business combinations, (b) our growth strategies, (c) our future financing plans, an d (d) our anticipated needs for working capital. Forward - looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words “may,” “will,” “should,” “expe ct, ” “anticipate,” “approximate,” “estimate,” “believe,” “intend,” “plan,” “budget,” “could,” “forecast,” “might,” “predict,” “shall” or “project,” or the negative of these words or other variations on these words or comparable terminology. Th is information may involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from the future results, performance, or achievements expres sed or implied by any forward - looking statements. These statements may be found in the Annual Report and in the Semiannual Report referred to immediately below. This presentation should be read in conjunction with the audited financial statements and related notes for the fiscal year e nde d April 30, 2018, contained in the Company’s Annual Report on Form 1 - K, as well as the condensed financial statements and related notes for the six months ended October 31, 2017, contained in the Company’s Semiannual Report on Form 1 - S A, as filed with the Securities and Exchange Commission on February 21, 2019 and January 30, 2018, respectively. Forward - looking statements are based on our current expectations and assumptions regarding our business, potential target busine sses, the economy and other future conditions. Because forward - looking statements relate to the future, by their nature, they are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward - looking statements as a result of various factors, including, without limitation, changes in local, regional, national or global political, economic, business, competi tiv e, market (supply and demand) and regulatory conditions and the following: ■ Our ability to effectively execute our business plan; ■ Our ability to manage our expansion, growth and operating expenses; ■ Our ability to evaluate and measure our business, prospects and performance metrics; ■ Our ability to compete and succeed in a highly competitive and evolving industry; ■ Our ability to respond and adapt to changes in technology and customer behavior; and ■ Our ability to protect our intellectual property and to develop, maintain and enhance a strong brand. We caution you therefore that you should not rely on any of these forward - looking statements as statements of historical fact or as guarantees or assurances of future performance. All forward - looking statements speak only as of the date of this presentation. We undertake no obligation to update any forward - looking statements or other information contained herein. Information regarding market and industry statistics contained in this presentation is included based on information availabl e t o us that we believe is accurate. It is generally based on academic and other publications that are not produced for purposes of securities offerings or economic analysis. Forecasts and other forward - looking information obtained from these sourc es are subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. Except as required by U.S. federal securities laws, we h ave no obligation to update forward - looking information to reflect actual results or changes in assumptions or other factors that could affect those statements. February 2019 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc . Alzamend ™ 2

Mission & Motto “To help the Alzheimer’s community through the support of research and the commercialization of preventions, treatments and cures of this devastating disease.” February 2019 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc . Join Alzamend™ in “Making Alzheimer’s Just a Memory ”! ™ Alzamend ™ 3

February 2019 Alzamend Neuro™ was founded in 2016 by Milton “Todd” Ault, III because of a lifelong goal to find a treatment/cure for Alzheimer’s and other neurodegenerative diseases that has plagued his family for generations. His unwavering passion and commitment led him to the University of South Florida (USF) Health Byrd Alzheimer’s Center and Research Institute, one the top 20 institutions in the nation for patented research and their portfolio of proprietary solutions. The Company has licensed two patented therapeutic compounds indicated for the treatment and prevention of Alzheimer’s disease. Our Story Alzamend ™ 4 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .

Our Team February 2019 Alzamend ™ Stephan Jackman Chief Executive Officer 20+ years multi - industry experience, specialized in Biotech and Pharmaceutical Kenneth S. Cragun Chief Financial Officer/Treasurer/Corporate Secretary 30+ Years multi - industry experience, including Biotech and Healthcare Gary R. Gottlieb Director, Bus. Dev. & Administration 35+ Years multi - industry experience, including Biotech and Healthcare Milton “Todd” Ault, III Founder/Executive Chairman 27+ years Financial Industry experience, seasoned Wall Street CEO & activist investor Philip E. Mansour Board of Directors 25+ years multi - industry experience, seasoned executive, manager & coach William B. Horne Board of Directors 25+ years Financial Industry experience, prior “Big 4” auditor & healthcare executive 5 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .

Scientific Advisory Board February 2019 Alzamend ™ Thomas M. Wisniewski, MD Director, NYU Langone’s Pearl I. Barlow Center for Memory Evaluation and Treatment 300+ Peer - Reviewed Medical Journal Publications ( 19 U.S. Patents Issued) Leads a Research Laboratory Continuously Funded by the National Institutes of Health for 20+ Years Yong Fan, MD Senior Consultant, A2Z Reg Solutions 10+ Years at the FDA/CBER/OTAT as a Quality reviewer and policy maker Knowledgeable of FDA’s Expedited Programs for Serious Conditions, new policies and current thinking. 6 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
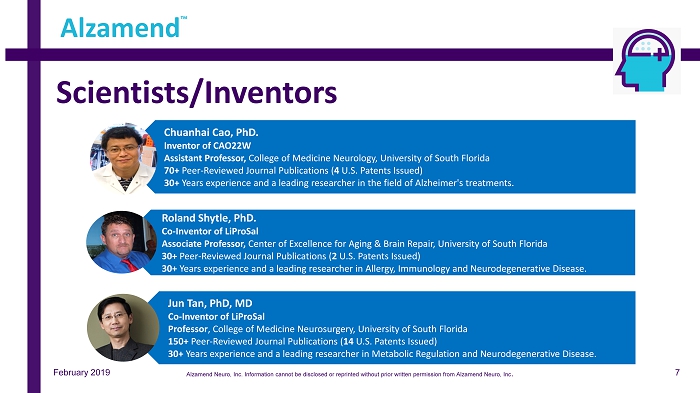
Scientists/Inventors February 2019 Alzamend ™ Chuanhai Cao, PhD. Inventor of CAO22W Assistant Professor, College of Medicine Neurology, University of South Florida 70+ Peer - Reviewed Journal Publications ( 4 U.S. Patents Issued) 30+ Years experience and a leading researcher in the field of Alzheimer's treatments. Roland Shytle, PhD. Co - Inventor of LiProSal Associate Professor, Center of Excellence for Aging & Brain Repair, University of South Florida 30+ Peer - Reviewed Journal Publications ( 2 U.S. Patents Issued) 30+ Years experience and a leading researcher in Allergy, Immunology and Neurodegenerative Disease. Jun Tan, PhD, MD Co - Inventor of LiProSal Professor , College of Medicine Neurosurgery, University of South Florida 150+ Peer - Reviewed Journal Publications ( 14 U.S. Patents Issued) 30+ Years experience and a leading researcher in Metabolic Regulation and Neurodegenerative Disease. 7 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .

Strategic Partner – TAMM Net, Inc. February 2019 Alzamend ™ Art Spaulding Founder and President, TAMM Net, Inc. 25+ years experience, including market research, reimbursement and regulatory. Donald P. Reitberg , Pharm.D . Pharmacologist 30+ year experience, including FDA briefing packages at FDA for Phases I - IV. Kenny Seaver , MS, RAC Regulatory Affairs, CMC 20+ Years experience in drug metabolism, pharmacokinetics, and CMC development Eve Del Rio, MD, PhD. Epidemiologist/Immunologist 30+ years experience, including pre - IND, INDs, pre - NDA, NDAs and BLAs. Gary W. Wolfe, PhD., DABT Pharmacologist/Toxicologist 30+ years experience preparing drug development plans for FDA approval. Michael Matthews, MS, RAC Regulatory Affairs 10+ years experience p reparing/reviewing technical docs required for INDs/NDAs. 8 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .

February 2019 We seek out and acquire early - stage, proprietary, innovative science residing on research benchtop shelves awaiting commercialization with the patent life ticking away. There is a 20+ year backlog of scientific discoveries that may be useful in human medicine. Alzamend™ finds these discoveries and fast - track develops them to bring treatments/cures to those suffering. Alzamend™ intends to secure funding by leveraging traditional channels, directed at accredited and institutional investors, and new financial regulations which permit direct marketing to the public. This approach will increase the speed, volume and breadth of capital raised while concurrently minimizing overhead costs. Our Approach Alzamend ™ 9 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .

Alzheimer’s Disease - The sixth leading cause of death in the United States Alzheimer’s disease (“AD”) is an irreversible, progressive brain disorder that slowly destroys memory and thinking skills, and eventually the ability to carry out the simplest tasks. In most people with Alzheimer’s, symptoms first appear in their early to mid - 60’s. Estimates vary, but experts suggest that more than 5.7 million Americans may have AD, considered by many as “the most feared” disease. Alzheimer’s has no current cure , but four treatments for symptoms are available today while research continues. Alzamend ™ February 2019 10 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .

Economic Burden – Alzheimer’s • The escalating Alzheimer’s epidemic has profound implications for government budgets: • Alzheimer’s is the most expensive disease in America, costing more than heart disease and cancer. • In 2018, caring for people with Alzheimer’s and other dementias will cost the United States an estimated $277 billion. Cumulatively between now and 2050, it will cost $20.2 trillion - two - thirds of which will be borne by Medicare and Medicaid. • One in every 5 dollars of Medicare spending is spent on people with Alzheimer’s and other dementias. • Despite the recent increased investment in Alzheimer’s research, funding still falls short of the need. • For fiscal year 2018, Congress provided $1.9 billion in Alzheimer’s research funding at the National Institutes of Health (NIH). • For every $9,700 Medicare and Medicaid spend caring for people with Alzheimer’s, the NIH spends only $100 on Alzheimer’s research. Alzamend ™ February 2019 $186 $202 $239 $310 $402 $506 $609 $750 2018 2020 2025 2030 2035 2040 2045 2050 Alzheimer’s Costs to Medicare And Medicaid (in billions of 2018 dollars) 11 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
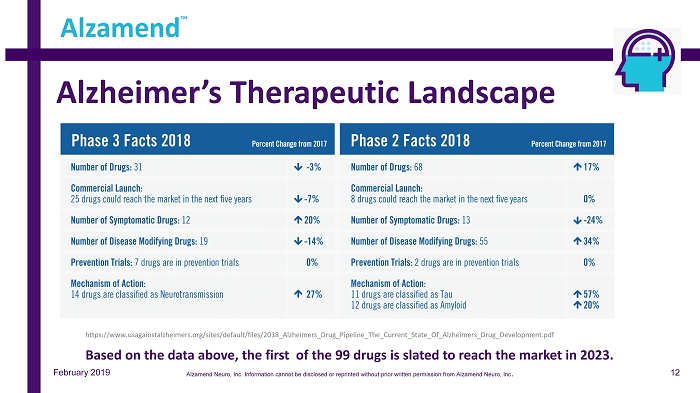
Alzheimer’s Therapeutic Landscape Alzamend ™ https://www.usagainstalzheimers.org/sites/default/files/2018_Alzheimers_Drug_Pipeline_The_Current_State_Of_Alzheimers_Drug_De vel opment.pdf February 2019 Based on the data above, the first of the 99 drugs is slated to reach the market in 2023. 12 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
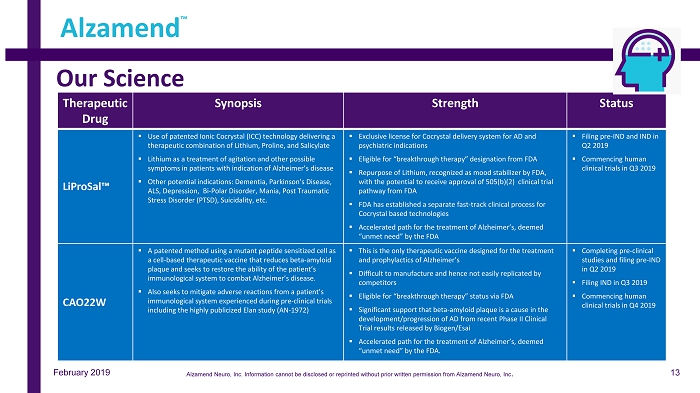
Our Science Alzamend ™ February 2019 Therapeutic Drug Synopsis Strength Status LiProSal™ ▪ Use of patented Ionic Cocrystal (ICC) technology delivering a therapeutic combination of Lithium, Proline, and Salicylate ▪ Lithium as a treatment of agitation and other possible symptoms in patients with indication of Alzheimer’s disease ▪ Other potential indications: Dementia, Parkinson’s Disease, ALS, Depression, Bi - Polar Disorder, Mania, Post Traumatic Stress Disorder (PTSD), Suicidality, etc. ▪ Exclusive license for Cocrystal delivery system for AD and psychiatric indications ▪ Eligible for “breakthrough therapy” designation from FDA ▪ Repurpose of Lithium, recognized as mood stabilizer by FDA, with the potential to receive approval of 505(b)(2) clinical trial pathway from FDA ▪ FDA has established a separate fast - track clinical process for Cocrystal based technologies ▪ Accelerated path for the treatment of Alzheimer’s, deemed “unmet need” by the FDA ▪ Filing pre - IND and IND in Q2 2019 ▪ Commencing human clinical trials in Q3 2019 CAO22W ▪ A patented method using a mutant peptide sensitized cell as a cell - based therapeutic vaccine that reduces beta - amyloid plaque and seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s disease. ▪ Also seeks to mitigate adverse reactions from a patient’s immunological system experienced during pre - clinical trials including the highly publicized Elan study (AN - 1972) ▪ This is the only therapeutic vaccine designed for the treatment and prophylactics of Alzheimer’s ▪ Difficult to manufacture and hence not easily replicated by competitors ▪ Eligible for “breakthrough therapy” status via FDA ▪ Significant support that beta - amyloid plaque is a cause in the development/progression of AD from recent Phase II Clinical Trial results released by Biogen/ Esai ▪ Accelerated path for the treatment of Alzheimer’s, deemed “unmet need” by the FDA. ▪ Completing pre - clinical studies and filing pre - IND in Q2 2019 ▪ Filing IND in Q3 2019 ▪ Commencing human clinical trials in Q4 2019 13 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
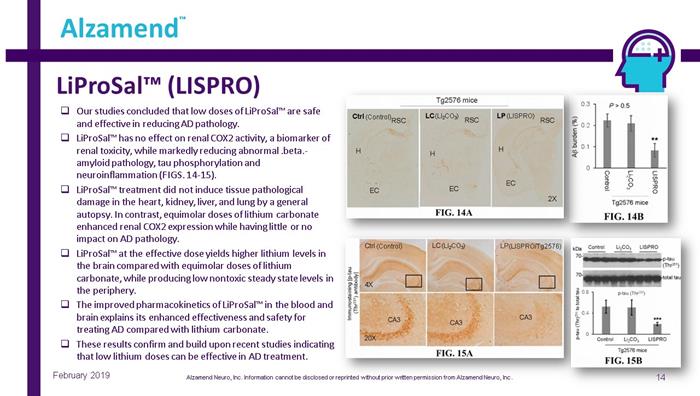
LiProSal™ (LISPRO) 14 Alzamend ™ February 2019 □ Our studies concluded that low doses of LiProSal™ are safe and effective in reducing AD pathology. □ LiProSal™ has no effect on renal COX2 activity, a biomarker of renal toxicity, while markedly reducing abnormal .beta. - amyloid pathology, tau phosphorylation and neuroinflammation (FIGS. 14 - 15). □ LiProSal™ treatment did not induce tissue pathological damage in the heart, kidney, liver, and lung by a general autopsy. In contrast, equimolar doses of lithium carbonate enhanced renal COX2 expression while having little or no impact on AD pathology. □ LiProSal™ at the effective dose yields higher lithium levels in the brain compared with equimolar doses of lithium carbonate, while producing low nontoxic steady state levels in the periphery. □ The improved pharmacokinetics of LiProSal™ in the blood and brain explains its enhanced effectiveness and safety for treating AD compared with lithium carbonate. □ These results confirm and build upon recent studies indicating that low lithium doses can be effective in AD treatment. (LI 2 CO 3 ) (LISPRO/Tg2576) (Control) (LI 2 CO 3 ) (Control) (LISPRO) Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
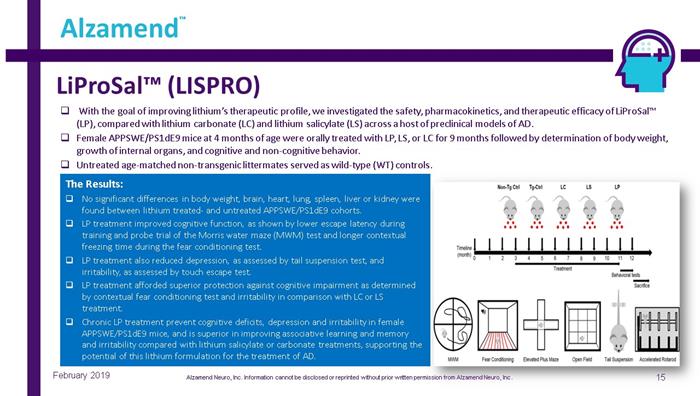
LiProSal™ (LISPRO) 15 Alzamend ™ February 2019 □ With the goal of improving lithium’s therapeutic profile, we investigated the safety, pharmacokinetics, and therapeutic effic ac y of LiProSal™ (LP), compared with lithium carbonate (LC) and lithium salicylate (LS) across a host of preclinical models of AD. □ Female APPSWE/PS1dE9 mice at 4 months of age were orally treated with LP, LS, or LC for 9 months followed by determination of bo dy weight, growth of internal organs, and cognitive and non - cognitive behavior. □ Untreated age - matched non - transgenic littermates served as wild - type (WT) controls. The Results: □ No significant differences in body weight, brain, heart, lung, spleen, liver or kidney were found between lithium treated - and untreated APPSWE/PS1dE9 cohorts. □ LP treatment improved cognitive function, as shown by lower escape latency during training and probe trial of the Morris water maze (MWM) test and longer contextual freezing time during the fear conditioning test. □ LP treatment also reduced depression, as assessed by tail suspension test, and irritability, as assessed by touch escape test. □ LP treatment afforded superior protection against cognitive impairment as determined by contextual fear conditioning test and irritability in comparison with LC or LS treatment. □ Chronic LP treatment prevent cognitive deficits, depression and irritability in female APPSWE/PS1dE9 mice, and is superior in improving associative learning and memory and irritability compared with lithium salicylate or carbonate treatments, supporting the potential of this lithium formulation for the treatment of AD. Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
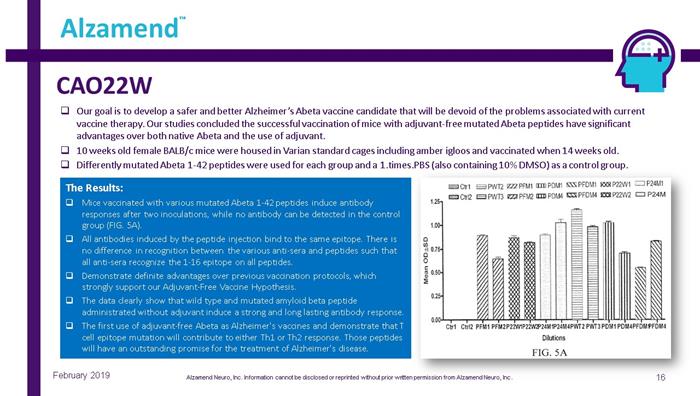
CAO22W 16 Alzamend ™ February 2019 □ Our goal is to develop a safer and better Alzheimer’s Abeta vaccine candidate that will be devoid of the problems associated wit h current vaccine therapy. Our studies concluded the successful vaccination of mice with adjuvant - free mutated Abeta peptides have signifi cant advantages over both native Abeta and the use of adjuvant. □ 10 weeks old female BALB/c mice were housed in Varian standard cages including amber igloos and vaccinated when 14 weeks old. □ Differently mutated Abeta 1 - 42 peptides were used for each group and a 1.times.PBS (also containing 10% DMSO) as a control group . The Results: □ Mice vaccinated with various mutated Abeta 1 - 42 peptides induce antibody responses after two inoculations, while no antibody can be detected in the control group (FIG. 5A). □ All antibodies induced by the peptide injection bind to the same epitope. There is no difference in recognition between the various anti - sera and peptides such that all anti - sera recognize the 1 - 16 epitope on all peptides. □ Demonstrate definite advantages over previous vaccination protocols, which strongly support our Adjuvant - Free Vaccine Hypothesis. □ The data clearly show that wild type and mutated amyloid beta peptide administrated without adjuvant induce a strong and long lasting antibody response. □ The first use of adjuvant - free Abeta as Alzheimer's vaccines and demonstrate that T cell epitope mutation will contribute to either Th1 or Th2 response. Those peptides will have an outstanding promise for the treatment of Alzheimer's disease. Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
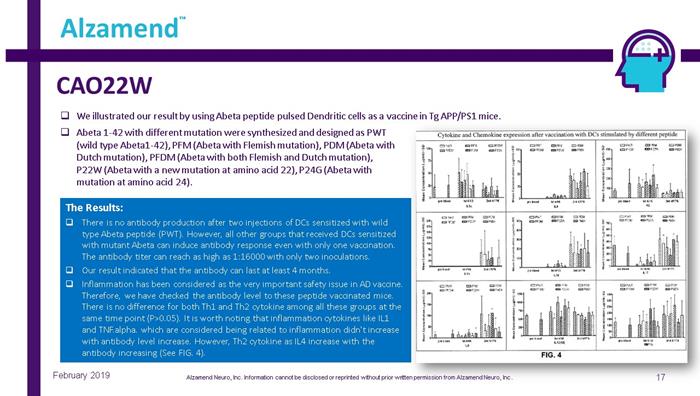
CAO22W 17 Alzamend ™ February 2019 □ We illustrated our result by using Abeta peptide pulsed Dendritic cells as a vaccine in Tg APP/PS1 mice. The Results: □ There is no antibody production after two injections of DCs sensitized with wild type Abeta peptide (PWT). However, all other groups that received DCs sensitized with mutant Abeta can induce antibody response even with only one vaccination. The antibody titer can reach as high as 1:16000 with only two inoculations. □ Our result indicated that the antibody can last at least 4 months. □ Inflammation has been considered as the very important safety issue in AD vaccine. Therefore, we have checked the antibody level to these peptide vaccinated mice. There is no difference for both Th1 and Th2 cytokine among all these groups at the same time point (P>0.05). It is worth noting that inflammation cytokines like IL1 and TNF.alpha . which are considered being related to inflammation didn't increase with antibody level increase. However, Th2 cytokine as IL4 increase with the antibody increasing (See FIG. 4). □ Abeta 1 - 42 with different mutation were synthesized and designed as PWT (wild type Abeta1 - 42), PFM (Abeta with Flemish mutation), PDM (Abeta with Dutch mutation), PFDM (Abeta with both Flemish and Dutch mutation), P22W (Abeta with a new mutation at amino acid 22), P24G (Abeta with mutation at amino acid 24). Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
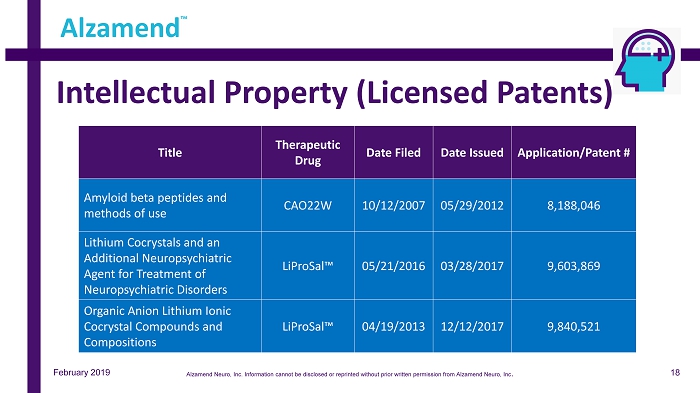
Intellectual Property (Licensed Patents) Alzamend ™ Title Therapeutic Drug Date Filed Date Issued Application/Patent # Amyloid beta peptides and methods of use CAO22W 10/12/2007 05/29/2012 8,188,046 Lithium Cocrystals and an Additional Neuropsychiatric Agent for Treatment of Neuropsychiatric Disorders LiProSal™ 05/21/2016 03/28/2017 9,603,869 Organic Anion Lithium Ionic Cocrystal Compounds and Compositions LiProSal™ 04/19/2013 12/12/2017 9,840,521 February 2019 18 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
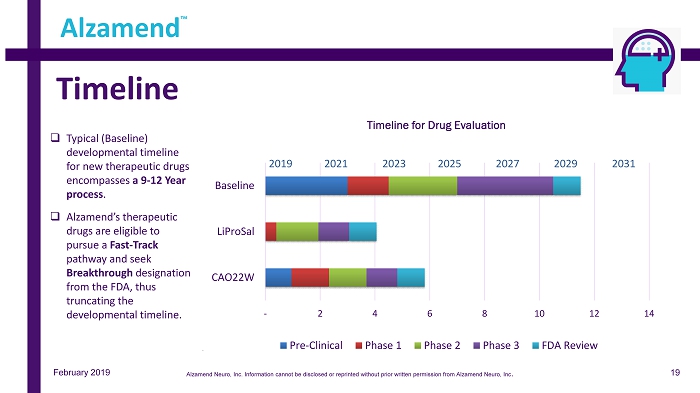
February 2019 Timeline Alzamend ™ - 2 4 6 8 10 12 14 CAO22W LiProSal Baseline Timeline for Drug Evaluation Pre-Clinical Phase 1 Phase 2 Phase 3 FDA Review 2019 20 21 2023 2025 2027 2029 2031 □ Typical (Baseline) developmental timeline for new therapeutic drugs encompasses a 9 - 12 Year process . □ Alzamend’s therapeutic drugs are eligible to pursue a Fast - Track pathway and seek Breakthrough designation from the FDA, thus truncating the developmental timeline. 19 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
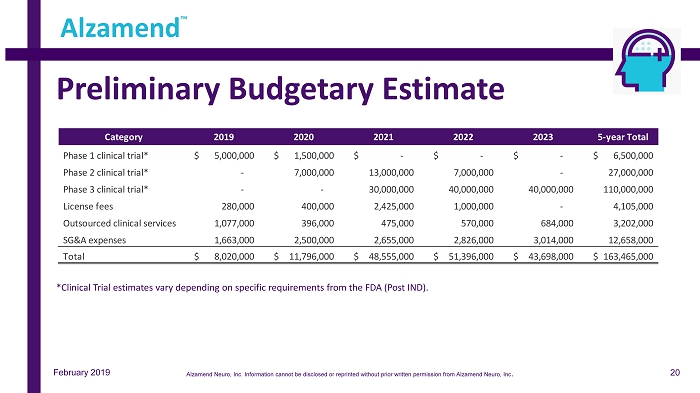
Preliminary Budgetary Estimate Alzamend ™ *Clinical Trial estimates vary depending on specific requirements from the FDA (Post IND). Category 2019 2020 2021 2022 2023 5-year Total Phase 1 clinical trial* 5,000,000$ 1,500,000$ -$ -$ -$ 6,500,000$ Phase 2 clinical trial* - 7,000,000 13,000,000 7,000,000 - 27,000,000 Phase 3 clinical trial* - - 30,000,000 40,000,000 40,000,000 110,000,000 License fees 280,000 400,000 2,425,000 1,000,000 - 4,105,000 Outsourced clinical services 1,077,000 396,000 475,000 570,000 684,000 3,202,000 SG&A expenses 1,663,000 2,500,000 2,655,000 2,826,000 3,014,000 12,658,000 Total 8,020,000$ 11,796,000$ 48,555,000$ 51,396,000$ 43,698,000$ 163,465,000$ 20 February 2019 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
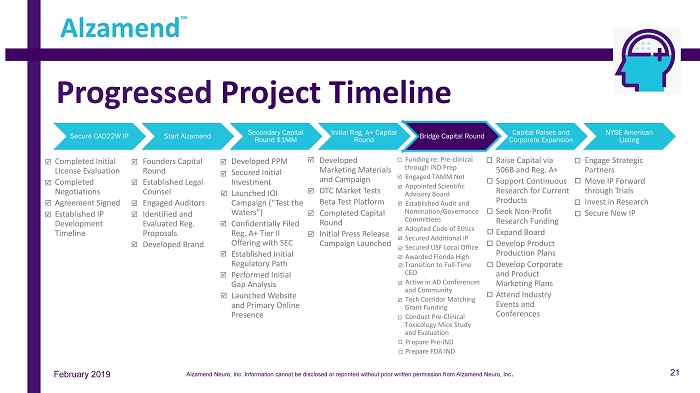
Secure CAO22W IP • Completed Initial License Evaluation • Completed Negotiations • Agreement Signed • Established IP Development Timeline Start Alzamend • Founders Capital Round • Established Legal Counsel • Engaged Auditors • Identified and Evaluated Reg. Proposals • Developed Brand Secondary Capital Round $1MM • Developed PPM • Secured Initial Investment • Launched IOI Campaign (“Test the Waters”) • Confidentially Filed Reg. A+ Tier II Offering with SEC • Established Initial Regulatory Path • Performed Initial Gap Analysis • Launched Website and Primary Online Presence Initial Reg. A+ Capital Round • Developed Marketing Materials and Campaign • DTC Market Tests • Beta Test Platform • Completed Capital Round • Initial Press Release Campaign Launched Bridge Capital Round • Funding re: Pre - clinical through IND Prep • Engaged TAMM Net • Appointed Scientific Advisory Board • Established Audit and Nomination/Governance Committees • Adopted Code of Ethics • Secured Additional IP • Secured USF Local Office • Awarded Florida High Transition to Full - Time CEO • Active in AD Conferences and Community • Tech Corridor Matching Grant Funding • Conduct Pre - Clinical Toxicology Mice Study and Evaluation • Prepare Pre - IND • Prepare FDA IND Capital Raises and Corporate Expansion • Raise Capital via 506B and Reg. A+ • Support Continuous Research for Current Products • Seek Non - Profit Research Funding • Expand Board • Develop Product Production Plans • Develop Corporate and Product Marketing Plans • Attend Industry Events and Conferences NYSE American Listing • Engage Strategic Partners • Move IP Forward through Trials • Invest in Research • Secure New IP Progressed Project Timeline Alzamend ™ February 2019 21 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
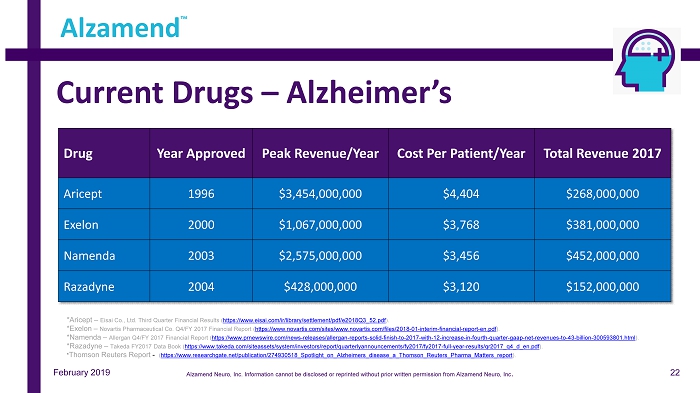
Current Drugs – Alzheimer’s Drug Year Approved Peak Revenue /Year Cost Per Patient/Year Total Revenue 2017 Aricept 1996 $3,454,000,000 $4,404 $268,000,000 Exelon 2000 $1,067,000,000 $3,768 $381,000,000 Namenda 2003 $2,575,000,000 $3,456 $452,000,000 Razadyne 2004 $428,000,000 $3,120 $152,000,000 Alzamend ™ *Aricept – Eisai Co., Ltd. Third Quarter Financial Results ( https://www.eisai.com/ir/library/settlement/pdf/e2018Q3_52.pdf ). *Exelon – Novartis Pharmaceutical Co. Q4/FY 2017 Financial Report ( https://www.novartis.com/sites/www.novartis.com/files/2018 - 01 - interim - financial - report - en.pdf ). *Namenda – Allergan Q4/FY 2017 Financial Report ( https://www.prnewswire.com/news - releases/allergan - reports - solid - finish - to - 2017 - with - 12 - increase - in - fourth - quarter - gaap - net - reven ues - to - 43 - billion - 300593801.html ). * Razadyne – Takeda FY2017 Data Book ( https://www.takeda.com/siteassets/system/investors/report/quarterlyannouncements/fy2017/fy2017 - full - year - results/qr2017_q4_d_en. pdf ). * Thomson Reuters Report - ( https://www.researchgate.net/publication/274930518_Spotlight_on_Alzheimers_disease_a_Thomson_Reuters_Pharma_Matters_report ). February 2019 22 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
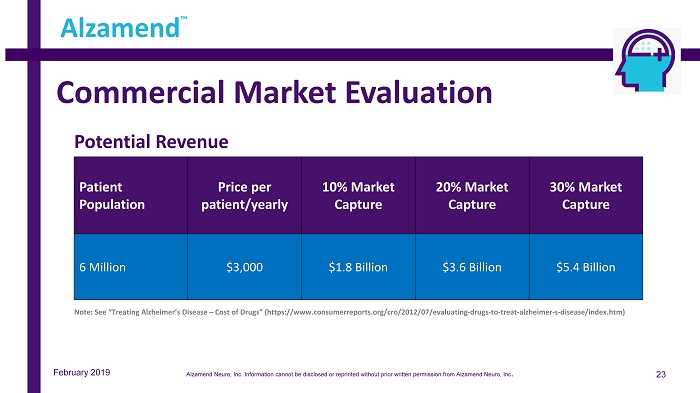
Commercial Market Evaluation 23 Alzamend ™ Note: See “Treating Alzheimer’s Disease – Cost of Drugs” (https://www.consumerreports.org/cro/2012/07/evaluating - drugs - to - treat - alzheimer - s - disease/index.htm) Patient Population Price per patient/yearly 10% Market Capture 20% Market Capture 30% Market Capture 6 Million $3,000 $1.8 Billion $3.6 Billion $5.4 Billion February 2019 Potential Revenue Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
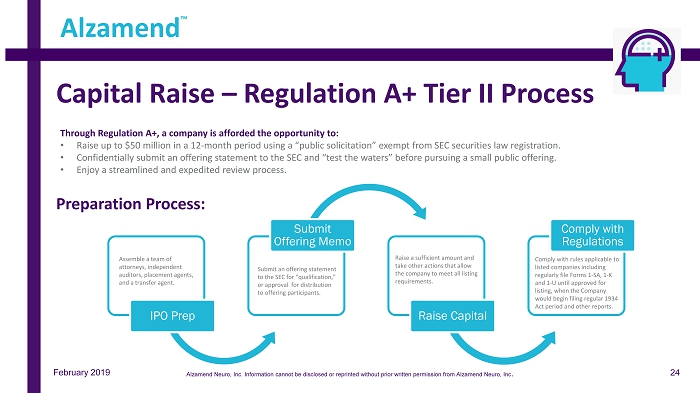
IPO Prep Submit Offering Memo Raise Capital Comply with Regulations Capital Raise – Regulation A+ Tier II Process Assemble a team of attorneys, independent auditors, placement agents, and a transfer agent. Submit an offering statement to the SEC for “qualification,” or approval for distribution to offering participants. Comply with rules applicable to listed companies including regularly file Forms 1 - SA, 1 - K and 1 - U until approved for listing, when the Company would begin filing regular 1934 Act period and other reports. Raise a sufficient amount and take other actions that allow the company to meet all listing requirements. Through Regulation A+, a company is afforded the opportunity to: • Raise up to $50 million in a 12 - month period using a “public solicitation” exempt from SEC securities law registration. • Confidentially submit an offering statement to the SEC and “test the waters” before pursuing a small public offering. • Enjoy a streamlined and expedited review process. Preparation Process: Alzamend ™ February 2019 24 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .
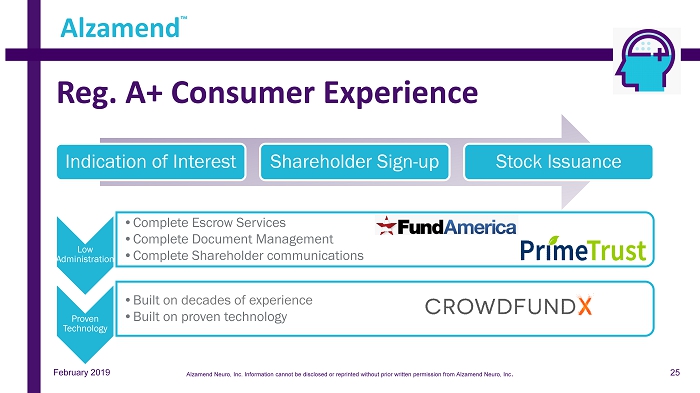
Reg. A+ Consumer Experience Low Administration • Complete Escrow Services • Complete Document Management • Complete Shareholder communications Proven Technology • Built on decades of experience • Built on proven technology Indication of Interest Shareholder Sign - up Stock Issuance Alzamend ™ February 2019 25 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .

Fighting Together to “Make Alzheimer’s Just a Memory”!™ Strategic Partners Alzamend ™ February 2019 26 Alzamend Neuro, Inc. Information cannot be disclosed or reprinted without prior written permission from Alzamend Neuro, Inc .

TM