
Exhibit 99.2

LiProSal™ Overview February 2019 TM

Safe Harbor Disclaimer This presentation contains forward - looking statements. All statements other than statements of historical fact are, or may be de emed to be, forward - looking statements. Such forward - looking statements include statements regarding, among others, (a) our expectations about possible business combinations, (b) our growth strategies, (c) our future financing plans, an d (d) our anticipated needs for working capital. Forward - looking statements, which involve assumptions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words “may,” “will,” “should,” “expe ct, ” “anticipate,” “approximate,” “estimate,” “believe,” “intend,” “plan,” “budget,” “could,” “forecast,” “might,” “predict,” “shall” or “project,” or the negative of these words or other variations on these words or comparable terminology. Th is information may involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from the future results, performance, or achievements expres sed or implied by any forward - looking statements. These statements may be found in the Annual Report and in the Semiannual Report referred to immediately below. This presentation should be read in conjunction with the audited financial statements and related notes for the fiscal year e nde d April 30, 2018, contained in the Company’s Annual Report on Form 1 - K, as well as the condensed financial statements and related notes for the six months ended October 31, 2017, contained in the Company’s Semiannual Report on Form 1 - S A, as filed with the Securities and Exchange Commission on February 21, 2019 and January 30, 2018, respectively. Forward - looking statements are based on our current expectations and assumptions regarding our business, potential target busine sses, the economy and other future conditions. Because forward - looking statements relate to the future, by their nature, they are subject to inherent uncertainties, risks, and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward - looking statements as a result of various factors, including, without limitation, changes in local, regional, national or global political, economic, business, competi tiv e, market (supply and demand) and regulatory conditions and the following: ■ Our ability to effectively execute our business plan; ■ Our ability to manage our expansion, growth and operating expenses; ■ Our ability to evaluate and measure our business, prospects and performance metrics; ■ Our ability to compete and succeed in a highly competitive and evolving industry; ■ Our ability to respond and adapt to changes in technology and customer behavior; and ■ Our ability to protect our intellectual property and to develop, maintain and enhance a strong brand. We caution you therefore that you should not rely on any of these forward - looking statements as statements of historical fact or as guarantees or assurances of future performance. All forward - looking statements speak only as of the date of this presentation. We undertake no obligation to update any forward - looking statements or other information contained herein. Information regarding market and industry statistics contained in this presentation is included based on information availabl e t o us that we believe is accurate. It is generally based on academic and other publications that are not produced for purposes of securities offerings or economic analysis. Forecasts and other forward - looking information obtained from these sourc es are subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. Except as required by U.S. federal securities laws, we h ave no obligation to update forward - looking information to reflect actual results or changes in assumptions or other factors that could affect those statements. February 2019 Confidential Alzamend ™ 2
LiProSal™ Per TAMM NET A novel drug delivery system/active pharmaceutical ingredient ionic co - crystal of lithium product (LiProSal™) that attenuates peak - to - trough concentrations via sustained - release properties yet additionally bolsters/stabilizes the ratio of brain - to - serum concentrations (enhances brain lithium exposure) could revolutionize the clinical utility of lithium therapy. The blood - brain barrier, restricting access of substances into the brain, occurs along all capillaries and consists of tight jun ctions around the capillaries that do not exist in normal circulation. Perhaps related to this barrier and as stated earlier, it is kno wn that lithium brain concentrations are only weakly correlated to blood lithium concentrations. Overcoming the blood - brain barrier is a daunting hurdle for pharmacologists. If brain lithium concentrations can be increased and/or be more reproducible/less variab le while the total body lithium burden can be decreased, the benefit - to - risk profile may be favorably improved for lithium treatments . The cocrystal of lithium formulation (LiProSal™) may be dose - sparing, thereby lowering total body lithium exposure while maintaining efficacy, and lowering the potential for adverse events. February 2019 Confidential Alzamend ™ 3 “Lower dosage of LiProSal™ will produce the same if not higher efficacy, minimize side effects (if any exists) and improve the safety profile of lithium, when compared to the current therapy on the market” . Hence, LiProSal™ can become the replacement for ALL lithium therapy on the market” .
The current target by Alzamend™ for an NDA exploits the enhanced absorption of ionic cocrystals of lithium (LiProSal™) into brain tissue, providing an opportunity to explore additional indications that may require relatively high brain lithium concentrati ons , such as for neurodegenerative disorders (Alzheimer’s, Parkinson’s, ALS, etc.). For these disorders, higher (or lower) brain l ith ium concentrations may be needed compared to those needed for mood disorders and so dose finding studies may be needed. If higher brain concentrations are needed for treatment of neurodegenerative disorders than mood disorders, systemic toxicity ma y only be avoidable by virtue of enhanced brain lithium distribution when using an ionic cocrystal lithium (LiProSal™) formulation, whereas traditional lithium dosage forms would have an unacceptable safety profile when achieving comparable brain lithium concentrations. If lower brain concentrations are needed for treatment of neurodegenerative disorders than mood disorders, systemic toxicity may not present when using an ionic cocrystal lithium formulation and so no therapeutic drug monitoring wou ld be needed. An ionic cocrystal lithium (LiProSal™) formulation may importantly expand the range of therapeutic categories amenable to lithium treatments, with enhanced safety. February 2019 Confidential Alzamend ™ 4 LiProSal™ Per TAMM NET “Our game plan is to go after Neurodegenerative disorders (Alzheimer) because of the fast - track designation associated with the diseases . Additionally, upon approval of LiProSal™ for Alzheimer’s, because we will have proven its enhanced safety, physicians will be able to prescribe it off - label for psychiatric disorders” .

LiProSal™ Regulatory Pathway Alzamend™ is pursuing a 505(b)(2) (Pages 6 - 8) regulatory approval pathway for LiProSal™. Currently, we are in the process of Putting together our portfolio for a Pre - IND meeting with the FDA. Upon completion, we will schedule said meeting with the FDA t o review and provide guidance. Three scenarios can occur via the pre - IND meeting; 1. The FDA will be in favor of our information and encourage us to apply for the IND. 2. The FDA will require additional information, which can be to be included in our IND application. 3. The FDA will require additional Preclinical testing in animals. We are working toward putting together a comprehensive package which will garner full support from the FDA with the encouragement to apply for the IND (Scenario #1). Post IND approval, we anticipate a robust Phase I Clinical Trial, adhering to all guidelines and recommendations established by the FDA, Tamm Net, and our Scientific Advisory Board. The results from Phase I testing will provide insight as to whether we will have to conduct separate Phase II and Phase III trials, skip certain steps , r educe patient count or conduct a combined Phase II/III trial. With proper funding, we anticipate a Pre - IND meeting and IND Approval in Q2, with Phase I to commence at the beginning of Q3. We anticipate Phase I to be completed within 3 - 6 months (pending agreement/approval by the FDA). Phase II or a potential Phase II/III combination may commence as early as Q4, 2019. February 2019 Confidential Alzamend ™ 5
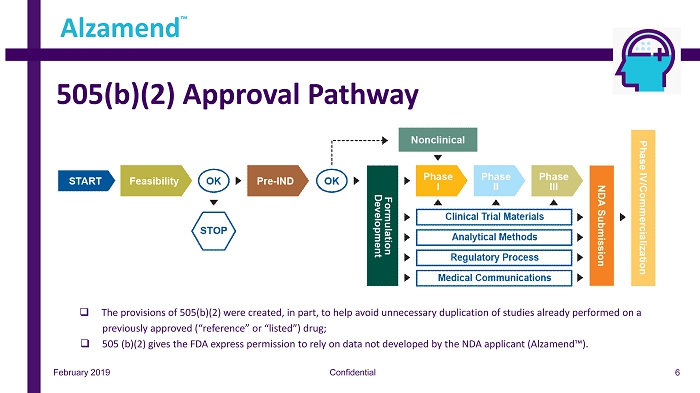
505(b)(2) Approval Pathway February 2019 Confidential Alzamend ™ 6 □ The provisions of 505 (b)( 2 ) were created, in part, to help avoid unnecessary duplication of studies already performed on a previously approved (“reference” or “listed”) drug ; □ 505 (b)( 2 ) gives the FDA express permission to rely on data not developed by the NDA applicant (Alzamend™) .

505(b)(2) Approval Pathway February 2019 Confidential Alzamend ™ 7 Benefits of 505(b)(2) 505(b)(2) is particularly valuable for pharmaceutical and generics companies looking to alleviate competitive forces in their environments while still wanting to benefit from a development process that eliminates most nonclinical studies as well as extensive safety and efficacy tests. ▪ Relatively low risk because of previous drug approval ▪ Lower cost, accelerated development due to fewer studies ▪ May qualify for three, five or seven years of market exclusivity ▪ Usually faster to product commercialization and to use by patients in need.

505(b)(2) Approval Pathway February 2019 Confidential Alzamend ™ 8 Phase I In certain instances, the 505(b)(2) pathway enables the Phase I process to be reduced to a single study. This study, known as a Phase I bridging study, is used to compare the human pharmacokinetic profile of the proposed drug product with that of the reference product (a clini cal bioequivalence study). Done properly, a bridging study allows a company to reference the established safety information for the original dru g. ▪ The specific type of Phase I bridging study for a 505(b)(2) product depends on the nature of the dose form and the reference pro duct. For an immediate - release oral dosage form, for example, the Phase I study is often a fasting, single - dose, crossover bioavailability/bioequivalen ce (BA/BE) study in healthy human volunteers in which the new drug product is compared with the reference product using pharmacokinetic assessments. Repe at - dose studies as well as those conducted in the fed state are sometimes required as well. ▪ In some cases, the requirement for an in vivo bioequivalence study can be waived via a formal request called a biowaiver. Phase II ▪ Certain 505(b)(2) development programs require no Phase II or Phase III studies (e.g., dosage form changes may rely on Phase I p harmacokinetic studies alone). ▪ In some 505(b)(2) NDAs, Phase II and Phase III studies can be combined. Phase III ▪ If a Phase III study is required for a 505(b)(2), such as when approval is sought for a product of a previously approved acti ve ingredient, only one study is often necessary. ▪ Fewer patients may be needed for 505(b)(2) product clinical trials due to the existing large exposure information available i n t he public literature or in the FDA’s databases.
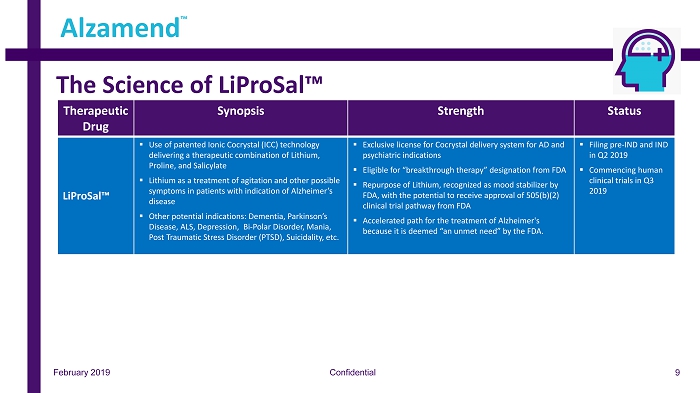
The Science of LiProSal™ Confidential Alzamend ™ February 2019 Therapeutic Drug Synopsis Strength Status LiProSal™ ▪ Use of patented Ionic Cocrystal (ICC) technology delivering a therapeutic combination of Lithium, Proline, and Salicylate ▪ Lithium as a treatment of agitation and other possible symptoms in patients with indication of Alzheimer’s disease ▪ Other potential indications: Dementia, Parkinson’s Disease, ALS, Depression, Bi - Polar Disorder, Mania, Post Traumatic Stress Disorder (PTSD), Suicidality, etc. ▪ Exclusive license for Cocrystal delivery system for AD and psychiatric indications ▪ Eligible for “breakthrough therapy” designation from FDA ▪ Repurpose of Lithium, recognized as mood stabilizer by FDA, with the potential to receive approval of 505(b)(2) clinical trial pathway from FDA ▪ Accelerated path for the treatment of Alzheimer's because it is deemed “an unmet need” by the FDA. ▪ Filing pre - IND and IND in Q2 2019 ▪ Commencing human clinical trials in Q3 2019 9
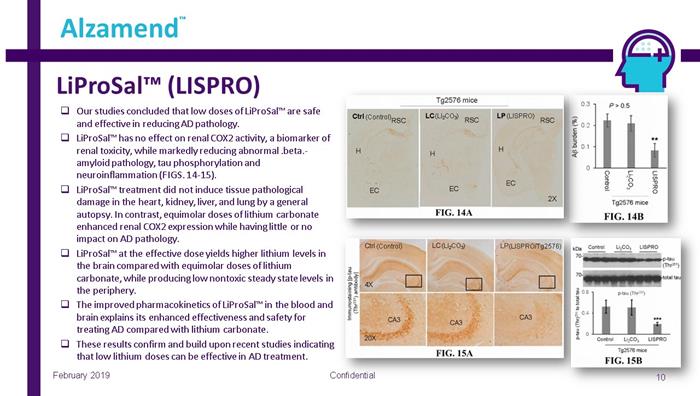
LiProSal™ (LISPRO) Confidential 10 Alzamend ™ February 2019 □ Our studies concluded that low doses of LiProSal™ are safe and effective in reducing AD pathology. □ LiProSal™ has no effect on renal COX2 activity, a biomarker of renal toxicity, while markedly reducing abnormal .beta. - amyloid pathology, tau phosphorylation and neuroinflammation (FIGS. 14 - 15). □ LiProSal™ treatment did not induce tissue pathological damage in the heart, kidney, liver, and lung by a general autopsy. In contrast, equimolar doses of lithium carbonate enhanced renal COX2 expression while having little or no impact on AD pathology. □ LiProSal™ at the effective dose yields higher lithium levels in the brain compared with equimolar doses of lithium carbonate, while producing low nontoxic steady state levels in the periphery. □ The improved pharmacokinetics of LiProSal™ in the blood and brain explains its enhanced effectiveness and safety for treating AD compared with lithium carbonate. □ These results confirm and build upon recent studies indicating that low lithium doses can be effective in AD treatment. (LI 2 CO 3 ) (LISPRO/Tg2576) (Control) (LI 2 CO 3 ) (Control) (LISPRO)
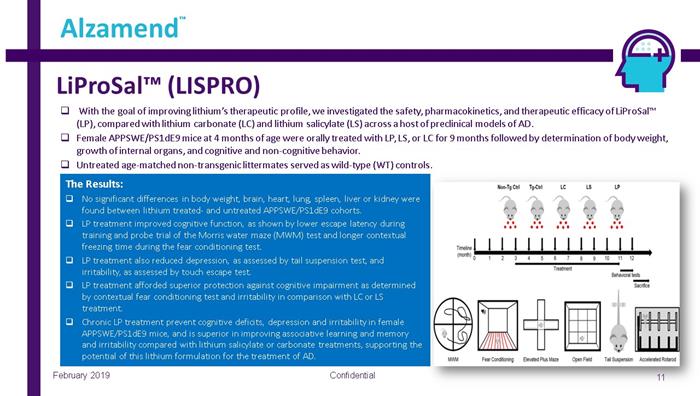
LiProSal™ (LISPRO) Confidential 11 Alzamend ™ February 2019 □ With the goal of improving lithium’s therapeutic profile, we investigated the safety, pharmacokinetics, and therapeutic effic ac y of LiProSal™ (LP), compared with lithium carbonate (LC) and lithium salicylate (LS) across a host of preclinical models of AD. □ Female APPSWE/PS1dE9 mice at 4 months of age were orally treated with LP, LS, or LC for 9 months followed by determination of bo dy weight, growth of internal organs, and cognitive and non - cognitive behavior. □ Untreated age - matched non - transgenic littermates served as wild - type (WT) controls. The Results: □ No significant differences in body weight, brain, heart, lung, spleen, liver or kidney were found between lithium treated - and untreated APPSWE/PS1dE9 cohorts. □ LP treatment improved cognitive function, as shown by lower escape latency during training and probe trial of the Morris water maze (MWM) test and longer contextual freezing time during the fear conditioning test. □ LP treatment also reduced depression, as assessed by tail suspension test, and irritability, as assessed by touch escape test. □ LP treatment afforded superior protection against cognitive impairment as determined by contextual fear conditioning test and irritability in comparison with LC or LS treatment. □ Chronic LP treatment prevent cognitive deficits, depression and irritability in female APPSWE/PS1dE9 mice, and is superior in improving associative learning and memory and irritability compared with lithium salicylate or carbonate treatments, supporting the potential of this lithium formulation for the treatment of AD.
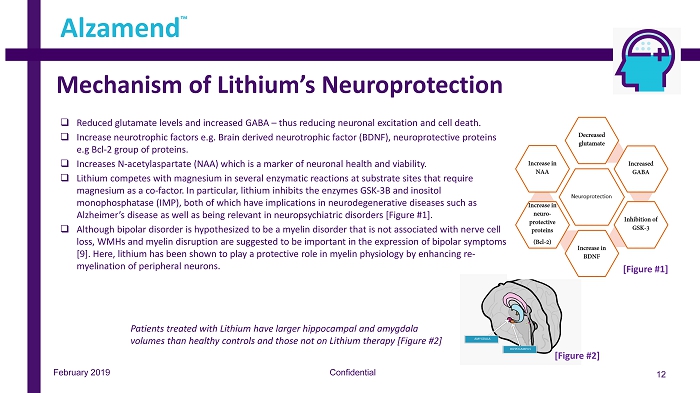
Mechanism of Lithium’s Neuroprotection Confidential 12 Alzamend ™ February 2019 □ Reduced glutamate levels and increased GABA – thus reducing neuronal excitation and cell death. □ Increase neurotrophic factors e.g. Brain derived neurotrophic factor (BDNF), neuroprotective proteins e.g Bcl - 2 group of proteins. □ Increases N - acetylaspartate (NAA) which is a marker of neuronal health and viability. □ Lithium competes with magnesium in several enzymatic reactions at substrate sites that require magnesium as a co - factor. In particular, lithium inhibits the enzymes GSK - 3B and inositol monophosphatase (IMP), both of which have implications in neurodegenerative diseases such as Alzheimer’s disease as well as being relevant in neuropsychiatric disorders [Figure #1]. □ Although bipolar disorder is hypothesized to be a myelin disorder that is not associated with nerve cell loss, WMHs and myelin disruption are suggested to be important in the expression of bipolar symptoms [9]. Here, lithium has been shown to play a protective role in myelin physiology by enhancing re - myelination of peripheral neurons. [Figure #1] Patients treated with Lithium have larger hippocampal and amygdala volumes than healthy controls and those not on Lithium therapy [Figure #2] [Figure #2]
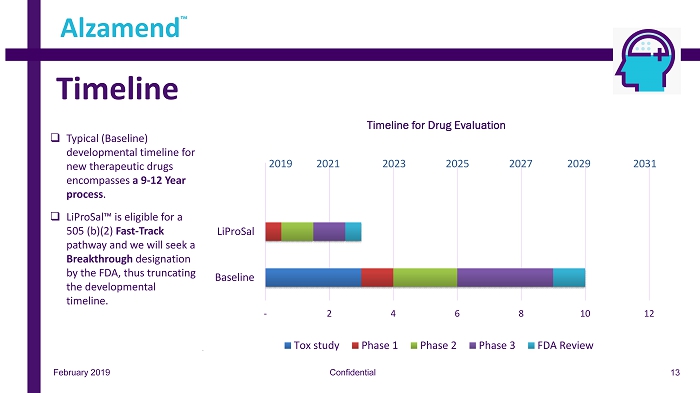
February 2019 Confidential Timeline Alzamend ™ - 2 4 6 8 10 12 Baseline LiProSal Timeline for Drug Evaluation Tox study Phase 1 Phase 2 Phase 3 FDA Review 2019 20 21 2023 2025 2027 2029 2031 □ Typical (Baseline) developmental timeline for new therapeutic drugs encompasses a 9 - 12 Year process . □ LiProSal™ is eligible for a 505 (b)(2) Fast - Track pathway and we will seek a Breakthrough designation by the FDA, thus truncating the developmental timeline. 13
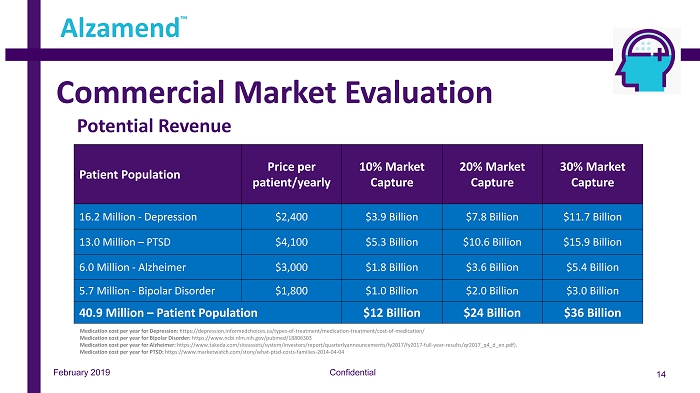
Commercial Market Evaluation Confidential 14 Alzamend ™ Note: See “Treating Alzheimer’s Disease – Cost of Drugs” (https://www.consumerreports.org/cro/2012/07/evaluating - drugs - to - treat - alzheimer - s - disease/index.htm) Patient Population Price per patient/yearly 10% Market Capture 20% Market Capture 30% Market Capture 16.2 Million - Depression $2,400 $3.9 Billion $7.8 Billion $11.7 Billion 13.0 Million – PTSD $4,100 $5.3 Billion $10.6 Billion $15.9 Billion 6.0 Million - Alzheimer $3,000 $1.8 Billion $3.6 Billion $5.4 Billion 5.7 Million - Bipolar Disorder $1,800 $1.0 Billion $2.0 Billion $3.0 Billion 40.9 Million – Patient Population $12 Billion $24 Billion $36 Billion February 2019 Potential Revenue Medication cost per year for Depression: https://depression.informedchoices.ca/types - of - treatment/medication - treatment/cost - of - medication/ Medication cost per year for Bipolar Disorder: https://www.ncbi.nlm.nih.gov/pubmed/18806303 Medication cost per year for Alzheimer: https://www.takeda.com/siteassets/system/investors/report/quarterlyannouncements/fy2017/fy2017 - full - year - results/qr2017_q4_d_en. pdf). Medication cost per year for PTSD: https://www.marketwatch.com/story/what - ptsd - costs - families - 2014 - 04 - 04

TM