Exhibit 6.6
STANDARD EXCLUSIVE LICENSE AGREEMENT
WITH SUBLICENSING TERMS
Agreement # LIC19050
This Agreement is made effective nunc pro tunc November 1, 2019, (the “Effective Date”) by and between the University of South Florida Research Foundation, Inc. (hereinafter called “ Licensor”), a nonstock, nonprofit Florida corporation, under Chapter 617 Florida Statutes, and a direct support organization of the University of South Florida (“University”) pursuant to section 1004.28 Florida Statutes and Alzamend Neuro Inc. (hereinafter called “Licensee”), a small corporation organized and existing under the laws of Delaware;
WHEREAS, University is the owner of certain inventions described in the “Licensed Patents” defined below (University Reference # 12B100);
WHEREAS, Licensor is the exclusive licensee of the Licensed Patents, and Licensor is willing to grant a license to Licensee under the Licensed Patents and Licensee desires a license to the Licensed Patents;
NOW, THEREFORE, in consideration of the mutual covenants and agreements set forth below, the parties covenant and agree as follows:
| Section 1 | Definitions |
| 1.1 | “Affiliate” means: (a) any person or entity which controls at least fifty percent (50%) of the equity or voting stock of the Licensee or (b) any person or entity fifty percent (50%) of whose equity or voting stock is owned or controlled by the Licensee or (c) any person or entity of which at least fifty percent (50%) of the equity or voting stock is owned or controlled by the same person or entity owning or controlling at least fifty percent (50%) of Licensee or (d) any entity in which any officer or employee is also an officer or employee of Licensee or any person who is an officer or employee of Licensee or (e) any other relationship as in fact, constitutes actual control. |
| 1.2 | “Development Plan” means the written report summarizing the development activities that are to be undertaken by the Licensee to bring Licensed Products and/or Licensed Processes to the market. The Development Plan is attached as Appendix A. |
| 1.3 | “Development Report” means a written account of Licensee’s progress under the Development Plan having at least the information specified on Appendix B to this Agreement, and shall be sent to the address specified on Appendix B . |
| 1.4 | “Investigator” means Drs. Roland (Doug) Shytle, Michael Zaworotko, Adam Smith and Naga Duggirala, while employed by Licensor. |
| 1.5 | “First Commercial Sale” means the first commercial sale, lease or other transfer, practice or disposition of any Licensed Product or Licensed Process for value in any country by Licensee or by a Sublicensee to a third party that is not a Licensee Affiliate or a Sublicensee. |
| 1.6 | “Know-How” means unpatented technology and/or information that was developed by the Investigator, including without limitation methods, processes, techniques, compounds, cell lines, materials, sequences, drawings, indications, data, results of tests, or studies, plans, and expertise, whether patentable or not, which relates specifically to the Licensed Patents and existing on the date hereof, only to the extent wholly owned and controlled by Licensor, except that, Know-How shall not include the Licensed Patents. |
| Page 1 |
| 1.7 | “Licensed Field” means the field of LiProSal (lithium co-crystal) for the treatment of Neurodegenerative Diseases excluding Alzheimer’s Disease. |
| 1.8 | “Licensed Patents” means all of the following Licensor intellectual property: |
| 1.8.1 | the patent(s)/patent application(s) identified on Schedule 1.8 hereto; |
| 1.8.2 | any and all United States and foreign patent applications claiming priority to any of the patent(s) and patent application(s) identified on Schedule 1 hereto (except that in the case of continuation-in-part application(s), only to the extent that the subject matter claimed in such continuation-in-part application(s) is supported under 35 U.S.C 112 in the patent(s)/patent application(s) identified on Schedule 1 hereto); and |
| 1.8.3 | any and all patents issuing from the patent applications identified in section 1.8.1 and 1.8.2, including, but not limited to, letters patents, patents of addition, reissues, re- examinations, extensions, restorations, and supplementary protection certificates; |
all to the extent owned or controlled by Licensor.
| 1.9 | “Licensed Product” and “Licensed Process” means: |
| 1.9.1 | In the case of a Licensed Product, any product or part thereof, on a country-by-country basis, that: |
| (a) | is covered in whole or in part by an issued, unexpired claim or a pending claim contained in the Licensed Patents, in any country in which such product is made, used, imported or sold; or |
| (b) | is manufactured by using a process that is covered in whole or in part by an issued, unexpired claim or a pending claim contained in the Licensed Patents, in any country in which any such process is used or in which any such product is used, imported, or sold; or |
| (c) | incorporates, utilizes, or was developed utilizing, Know-How or that is manufactured using Know-How or using a process developed using Know-How. |
| 1.9.2 | In the case of a Licensed Process, any process, on a country-by-country basis, that: |
| (a) | is covered in whole or in part by an issued, unexpired claim or a pending claim contained in the Licensed Patents in any country in which such process is practiced; or |
| (b) | incorporates, utilizes, or was developed utilizing, Know-How. |
| 1.10 | “Licensed Territory” means worldwide. |
| 1.11 | “Net Sales” means the total dollar amount invoiced on sales of Licensed Product and/or Licensed Processes by Licensee, Sublicensee or Affiliates. Total amount invoiced may include only promotional discounts allowed in amounts customary in the trade. |
| 1.12 | “Patent Challenge” means a challenge to the validity, patentability, enforceability and/or non-infringement of any of the Licensed Patents or otherwise opposing any of the Licensed Patents. |
| Page 2 |
| 1.13 | “Sublicense” means, an agreement or series of related agreements to, directly or indirectly, sublicense, grant any other right with respect to, or agree not to assert, any right licensed to Licensee under this Agreement. An agreement that is described in this definition is a Sublicense whether or not it is called a “sublicense” and whether or not it is included in a stand-alone document or is part of series of agreements establishing a broader collaboration, development, asset purchase, joint venture agreement or other arrangement. |
| 1.14 | “Sublicensee” means any third party to whom Licensee grants a Sublicense. |
| Section 2 | Grant |
| 2.1 | License. |
| 2.1.1 | License Under Licensed Patents and Know-How Subject to the terms of this Agreement, Licensor hereby grants to Licensee: a) a royalty- bearing, exclusive license, limited to the Licensed Field and the Licensed Territory, under the Licensed Patents to make, have made, develop, use, lease, import, export, offer to sell, sell and have sold Licensed Products and/or Licensed Processes, and b) a royalty bearing, non-exclusive license, limited to the Licensed Field and the Licensed Territory, under the Know-How to make, have made, develop, use, lease, import, export, offer to sell, sell and have sold Licensed Products and/or Licensed Processes. Licensor reserves to itself, University, and to all nonprofit entities with which it collaborates the right under the Licensed Patents to make, have made, develop, import and use Licensed Products and Licensed Processes solely for their internal research, clinical and educational purposes. In addition, Licensor reserves to itself and University, as well as to all non-profit research institutions with which it collaborates, the right to use materials that might be covered under Licensed Patents solely for their internal research, educational, and clinical purposes and to meet all applicable governmental and peer review journal requirements governing the transfer of materials. |
| 2.1.2 | The license granted hereunder shall not be construed to confer any rights upon Licensee by implication, estoppel, or otherwise as to any technology not part of the Licensed Patents in the specified Licensed Field and specified Licensed Territory. |
| 2.2 | Sublicense. |
| 2.2.1 | Licensee may grant written Sublicenses under the Licensed Patents to third parties upon Licensor’s approval, which approval shall not be unreasonably or untimely withheld. Any agreement granting a Sublicense shall state that the Sublicense is subject to the terms and conditions of this Agreement and to the termination of this Agreement. Licensee shall have the same responsibility for the activities of any Sublicensee or Affiliate as if the activities were directly those of Licensee. |
| 2.2.2 | Licensee shall provide Licensor with an unredacted copy of each Sublicense agreement (and in the case of a series of related agreements, all such related agreements) and any subsequent amendments which transfers intellectual property rights granted hereunder, at least thirty (30) days prior to the execution of the Sublicense agreement. Licensee shall also provide Licensor with copies of any Sublicensee milestone and royalty reports. |
| Page 3 |
| 2.2.3 | In the event that Licensor notifies Licensee in writing of a third party’s interest in a market or territory which Licensee is not addressing at the time of receipt of the notice, Licensee shall respond to Licensor in writing within thirty (30) days of receipt of such notice to inform Licensor whether Licensee intends to pursue the market or territory. If in such response, Licensee elects to forego the market or territory, Licensor may terminate in said market or territory the license granted in 2.1.1. If, in such response, Licensee elects to pursue the market or territory, Licensee shall provide Licensor with such response a revised Development Plan that addresses said market or territory. |
| Section 3 | Due Diligence |
| 3.1 | Development. |
| 3.1.1 | Licensee agrees to and warrants that: |
| (a) | it has, or will obtain, the expertise necessary to independently evaluate the inventions of the Licensed Patents and Know-How; |
| (b) | it will actively and diligently pursue the Development Plan, (see Appendix A) to the end that the inventions of the Licensed Patents will be utilized to provide Licensed Products and/or Licensed Processes for sale in the retail market within the Licensed Field; |
| (c) | it will diligently develop markets for Licensed Products and Licensed Processes; |
| (d) | and, until the date of First Commercial Sale of Licensed Products or Licensed Processes, it will supply Licensor with a written Development Report annually within fifteen (15) days after the end of the calendar year (see Appendix B ). |
| 3.1.2 | Licensee agrees that the First Commercial Sale of products to the retail customer shall occur on or before July 1, 2027 or Licensor shall have the right to terminate this Agreement pursuant to Section 9.3 hereto. In addition, Licensee will meet the milestones shown in Appendix D or Licensor shall have the right to terminate this Agreement pursuant to Section 9.3. Licensee will notify Licensor in writing as each milestone is met. |
| 3.1.3 | Upon written request by Licensee to negotiate extensions of any milestones or due dates set forth in Appendix D, such request to be received by Licensor no less than ninety (90) days prior to any of the due dates subject of such request, set forth in this Section 3.1.3, such request fully describing Licensee’s diligent efforts to achieve the milestone required to be met by such due date, Licensor shall consider in good faith such requests. Upon granting such request, Licensor and Licensee shall negotiate such extensions in good faith. |
| 3.1.4 | University’s policies may require approval of clinical trials involving technology invented by Licensor. Accordingly, Licensee will notify Licensor prior to commencing any clinical trials at the University’s facility or any affiliated medical facilities. |
| 3.1.5 | Every year Licensor is required to report on statistics that are relevant to growth of businesses in Florida. On January 31 and July 31 of each year, Licensee shall provide to Licensor a report that includes: the current number of employees in Florida, the total number of employees, information about whether Licensee has gone public or been acquired, detail on the amount and sources of funding, any new products that have been introduced to the market, the number of employees who are University graduates, and the number of University interns for the period since the last report was received. This specific information will be held in confidence and provided in the aggregate. No information obtained under this Section 3.1.5 will be identified as being connected with Licensee absent agreement of the Licensee. |
| Page 4 |
| Section 4 | Payments |
| 4.1 | License Issue Fee. |
Licensee agrees to pay Licensor a License Issue Fee of ten thousand Dollars ($10,000.00) due on the first anniversary of the Effective Date.
| 4.2 | Intentionally Omitted |
| 4.3 | Royalty. |
Royalty on Licensed Patents. In addition to the Section 4.1 License Issue Fee, Licensee agrees to pay to Licensor as earned royalties a royalty calculated as a percentage of Net Sales. The royalty is deemed earned as of the earlier of the date the Licensed Product and/or Licensed Process is actually sold and paid for, the date an invoice is sent by Licensee or its Sublicensee, or the date a Licensed Product and/or Licensed Process is transferred to a third party. Licensee shall pay to Licensor royalties as follows:
| (i) | three percent (3%) for Net Sales of Licensed Products, for each product, on a country- by-country basis, as defined by Sections 1.7.1 (a), and 1.7.1(b); and |
| (ii) | three percent (3%) for Net Sales of Licensed Processes, for each process, on a country-by-country basis, as defined by Section 1.7.2 (a); and |
| (iii) | two percent (2%) for Net Sales of Licensed Products and Licensed Processes sold during a period of regulatory exclusivity as defined by the appropriate regulatory body for such Licensed Product or Licensed Process in the country in which such Licensed Product or Licensed Process is sold. |
Royalties shall be payable until the later, on a country-by-country basis, of (i) the expiration of the last-to-expire Licensed Patents, (ii) the expiration of any applicable regulatory exclusivity period in such country, and (iii) ten (10) years from the First Commercial Sale of a Licensed Product or Licensed Process in such country in which the Licensed Product or Licensed Process is sold.
| Page 5 |
| 4.4 | Other Payments. |
| 4.4.1 | Licensee agrees to pay Licensor minimum royalty payments, as follows: |
| Payment | Year |
| $ 15,000.00 | 2023 |
| $ 30,000.00 | 2024 |
| $ 50,000.00 | 2025; and every year thereafter, for the life of this Agreement. |
The minimum royalty for a given year shall be due in advance and shall be paid in quarterly installments on March 31, June 30, September 30, and December 31 for the following quarter. Any minimum royalty paid in a calendar year will be credited against the earned royalties for that calendar year. It is understood that the minimum royalties will be applied to earned royalties on a calendar year basis, and that sales of Licensed Products and/or Licensed Processes requiring the payment of earned royalties made during a prior or subsequent calendar year shall have no effect on the annual minimum royalty due Licensor for other than the same calendar year in which the royalties were earned.
| 4.4.2 | In addition to all other payments required under this Agreement, Licensee agrees to pay Licensor milestone payments, as follows: |
| Payment | Event |
| $30,000.00 | Upon First Pre-IND Meeting |
| $50,000.00 | IND Filing |
| $150,000.00 | Upon first dosing of a patient in a clinical trial |
| $400,000.00 | Upon Completion of first Clinical Trial |
| $1,000,000.00 | Upon first patient treated in a Phase III Clinical Trial |
| $8,000,000.00 | Upon FDA Approval |
Licensee is entering into multiple license agreements with Licensor related to USF Technology 12B100, also referred to as the LiProSal product. For the avoidance of doubt, it is understood and agreed that only one of each such milestone payments shall be payable as between LIC19050 and LIC19051 ..
Sublicenses. In respect to Sublicenses granted by Licensee under 2.2.1 above, Licensee shall pay to Licensor an amount equal to what Licensee would have been required to pay to Licensor had Licensee sold the amount of Licensed Product or Licensed Process sold by such Sublicensee. In addition, if Licensee receives any fees, minimum royalties, milestone payments, or other payments arising from the Sublicense, and such payments are not earned royalties as defined in Section 4.3 above, then Licensee shall pay Licensor fifty percent (50%) of such payments within thirty (30) days of receipt thereof. Such payments shall not be allocated, off-set or otherwise reduced as a result of including rights other than those licensed hereunder in such permitted written Sublicense. Licensee shall not receive from Sublicensees anything of value in lieu of cash payments in consideration arising from any Sublicense under this Agreement without the express prior written permission of Licensor.
| Page 6 |
| 4.5 | Accounting for Payments. |
| 4.5.1 | Amounts owing to Licensor under Section 4.3 shall be paid on a quarterly basis after the amount of minimum royalties paid is exceeded, with such amounts due and received by Licensor on or before the thirtieth day following the end of the calendar quarter ending on March 31, June 30, September 30 or December 31 in which such amounts were earned. All royalties owing with respect to Net Sales stated in currencies other than U.S. dollars shall be converted at the rate shown in the Federal Reserve Noon Valuation - Value of Foreign Currencies on the day preceding the payment due date. |
| 4.5.2 | Any amounts which remain unpaid after the date they are due to Licensor shall accrue interest from the due date at the rate of 1.5% per month. However, in no event shall this interest provision be construed as a grant of permission for any payment delays. Licensee shall also be responsible for repayment to Licensor of any attorney, collection agency, or other out-of-pocket Licensor expenses required to collect overdue payments due under this Section 4 or any other applicable Section of this Agreement. |
| 4.5.3 | Except as otherwise directed, all amounts owing to Licensor under this Agreement shall be paid in U.S. dollars to Licensor at the following address: |
USF Research Foundation Attn: Business Manager
3802 Spectrum Blvd, Suite 100
Tampa, Florida 33612.
| 4.5.4 | A certified full accounting statement showing how any amounts payable to Licensor under Section 4 have been calculated shall be submitted to Licensor on the date of each such payment. In addition to being certified, such accounting statements shall contain a written representation signed by an executive officer of Licensee that states that the statements are true, accurate, and fairly represent all amounts payable to Licensor pursuant to this Agreement. For earned royalties, such accounting shall be on a per- country and product line, model or trade name basis and shall be summarized on the form shown in Appendix C – Licensor Royalty Report of this Agreement. For earned royalties, in the event no payment is owed to Licensor because the amount of minimum royalties paid has not been exceeded or otherwise, an accounting demonstrating that fact shall be supplied to Licensor. |
| 4.5.5 | Licensor is exempt from paying income taxes under U.S. law. Therefore, all payments due under this Agreement shall be made without deduction for taxes, assessments, or other charges of any kind which may be imposed on Licensor by any government outside of the United States or any political subdivision of such government with respect to any amounts payable to Licensor pursuant to this Agreement. All such taxes, assessments, or other charges shall be assumed by Licensee. |
| Page 7 |
| Section 5 | Certain Warranties and Disclaimers of Licensor |
| 5.1 | Licensor warrants that, except as otherwise provided under Section 17.1 of this Agreement with respect to U.S. Government interests, it is the owner or exclusive licensee of the Licensed Patents or otherwise has the right to grant the licenses granted to Licensee in this Agreement. However, nothing in this Agreement shall be construed as: |
| (a) | a warranty or representation by Licensor as to the validity or scope of any right included in the Licensed Patents; |
| (b) | a warranty or representation that anything made, used, sold or otherwise disposed of under the license granted in this Agreement will or will not infringe patents of third parties; |
| (c) | an obligation to bring or prosecute actions or suits against third parties for infringement of Licensed Patents; |
| (d) | an obligation to furnish any services other than those specified in this Agreement; or |
| (e) | a warranty or representation by Licensor that it will not grant licenses to others to make, use or sell products not covered by the claims of the Licensed Patents which may be similar and/or compete with products made or sold by Licensee. |
| 5.2 | Licensee warrants that it has the power and authority to enter into and perform its obligations under this Agreement and that the execution of this Agreement by it has been duly and validly authorized by all necessary corporate action and its obligations under this Agreement are valid and binding and enforceable against it in accordance with their terms. |
| 5.3 | EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT, LICENSOR MAKES NO REPRESENTATIONS AND EXTENDS NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, AND VALIDITY OF PATENT RIGHTS CLAIMS, ISSUED OR PENDING. LICENSOR ASSUMES NO RESPONSIBILITIES WHATSOEVER WITH RESPECT TO USE, SALE, OR OTHER DISPOSITION BY LICENSEE, ITS SUBLICENSEE(S), OR THEIR VENDEES OR OTHER TRANSFEREES OF PRODUCT INCORPORATING OR MADE BY USE OF INVENTIONS LICENSED UNDER THIS AGREEMENT. |
| Section 6 | Record Keeping |
| 6.1 | Licensee and its Sublicensee(s) shall keep books and records sufficient to verify the accuracy and completeness of Licensee’s and its Sublicensee(s)’s accounting referred to above, including without limitation, inventory, purchase and invoice records, manufacturing records, sales analysis, general ledgers, financial statements, and tax returns relating to the Licensed Products and/or Licensed Processes. Such books and records shall be preserved for a period not less than six years after they are created or as required by federal law, both during and after the term of this Agreement. |
| 6.2 | Licensee and its Sublicensee(s) shall take all steps necessary so that Licensor may, within thirty (30) days of its written request, audit, review and/or copy all of the books and records at a single U.S. location to verify the accuracy of Licensee’s and its Sublicensee(s)’s accounting. Such review may be performed by any authorized employees of Licensor as well as by any attorneys and/or accountants designated by Licensor, upon reasonable notice and during regular business hours. If a deficiency with regard to any payment hereunder is determined, Licensee and its Sublicensee(s) shall pay the deficiency within thirty (30) days of receiving notice thereof along with applicable interest as described in Section 4.5.1. If a royalty payment deficiency for a calendar year exceeds three percent (3%) of the royalties paid for that year, then Licensee and its Sublicensee(s) shall be responsible for paying Licensor’s out-of-pocket expenses incurred with respect to such review. |
| 6.3 | At any time during the term of this Agreement, Licensor may request in writing that Licensee verify the calculation of any past payments owed to Licensor through the means of a self-audit. Within ninety (90) days of the request, Licensee shall complete a self-audit of its books and records to verify the accuracy and completeness of the payments owed. Within thirty (30) days of the completion of the self-audit, Licensee shall submit to Licensor a report detailing the findings of the self-audit and the manner in which it was conducted in order to verify the accuracy and completeness of the payments owed. If Licensee has determined through its self- audit that there is any payment deficiency, Licensee shall pay Licensor the deficiency along with applicable interest under Section 4.5.1 with the submission of the self-audit report to Licensor. |
| Page 8 |
| Section 7 | Patent Prosecution |
| 7.1 | Licensor shall be solely responsible for preparing, filing, prosecuting and maintaining the Licensed Patents using counsel of its choice. Licensor shall provide Licensee with copies of all documents sent to and received from the United States Patent and Trademark Office and foreign patent offices relating to Licensed Patents. Licensee agrees to keep such information confidential. Licensor shall provide Licensee with a reasonable opportunity to comment on the preparation, prosecution and maintenance of the Licensed Patents and will consider Licensee’s comments in good faith. |
| 7.2 | Intentionally Omitted |
| 7.3 | Licensee shall be responsible for and pay all costs and expenses incurred by Licensor related to the preparation, filing, prosecution (including interferences), issuance, maintenance, defense (including oppositions) and reporting of the Licensed Patents subsequent to and separate of those expenses cited in Section 7.2 within thirty (30) days of receipt of an invoice from Licensor . It shall be the responsibility of Licensee to keep Licensor fully apprised of the “small entity” status of Licensee and all Sublicensees with respect to the U.S. patent laws and with respect to the patent laws of any other countries, if applicable, and to inform Licensor of any changes in writing of such status, within thirty (30) days of any such change. In the event that additional licenses are granted to licensees for alternate fields-of-use, patent expenses associated with Licensed Patents will be divided proportionally between the number of existing licensees. In the case of foreign patent protection, if Licensee gives sixty (60) days notice that it intends to decline to reimburse Licensor for patent expenses for any Licensed Patent in any particular country, then the license granted hereunder respecting such Licensed Patent shall terminate after such sixty (60) days and Licensee relinquishes the right to commercialize Licensed Products in the specified country. |
| Section 8 | Infringement and Invalidity |
| 8.1 | Licensee shall inform Licensor promptly in writing of any alleged infringement of the Licensed Patents by a third party and of any available evidence thereof. |
| 8.2 | During the term of this Agreement, Licensor shall have the right, but shall not be obligated, to prosecute at its own expense any such infringements of the Licensed Patents. If Licensor prosecutes any such infringement, Licensee agrees that Licensor may include Licensee as a co- plaintiff in any such suit, without expense to Licensee. |
| 8.3 | If within six (6) months after having been notified of any alleged infringement, Licensor shall have been unsuccessful in persuading the alleged infringer to desist and shall not have brought an infringement action against the alleged infringer, or if Licensor shall notify Licensee at any time prior thereto of its intention not to bring suit against the alleged infringer, then, and in those events only, Licensee shall have the right, but shall not be obligated, to prosecute at its own expense any infringement of the Licensed Patents, and Licensee may, for such purposes, use the name of Licensor as party plaintiff. No settlement, consent judgment or other voluntary final disposition of the suit may be entered into without the consent of Licensor, which consent shall not be unreasonably withheld. Licensee shall indemnify Licensor against any order for costs that may be made against Licensor in such proceedings. |
| Page 9 |
| 8.4 | In the event that a declaratory judgment action is brought against Licensor or Licensee by a third party alleging invalidity, unpatentability, unenforceability, or non-infringement of the Licensed Patents, Licensor, at its option, shall have the right within twenty (20) days after commencement of such action to take over the sole defense of the action at its own expense. If Licensor does not exercise this right, Licensee shall be responsible for the sole defense of the action at Licensee’s sole expense, subject to Sections 8.5 and 8.6. |
| 8.5 | In the event that Licensee shall undertake the enforcement by litigation and/or defense of the Licensed Patents by litigation, Licensor shall have the right, but not the obligation, to voluntarily join such litigation, represented by its own counsel at its own expense. In the event that Licensor or Licensee shall undertake the enforcement by litigation and/or defense of the Licensed Patents by litigation, any recovery of damages by Licensor or Licensee for any such suit shall be applied first in satisfaction of any unreimbursed expenses and legal fees of Licensor relating to the suit, and next toward reimbursement of any unreimbursed expenses and legal fees of Licensee relating to the suit. The balance remaining from any such recovery shall be divided equally between Licensee and Licensor. |
| 8.6 | In any suit in which either party is involved to enforce or defend the Licensed Patents pursuant to this Agreement, the other party hereto shall, at the request and expense of the party initiating such suit, cooperate in all respects and, to the extent possible, have its employees testify when requested and make available relevant records, papers, information, samples, specimens, and the like. |
| 8.7 | In the event Licensee, its Affiliate or Sublicensee brings a Patent Challenge against Licensor, or assists another party in bringing a Patent Challenge against Licensor, unless and until Licensor terminates this Agreement pursuant to Section 9, Licensee shall continue to pay royalties and make other payments pursuant to this Agreement with respect to the contested Licensed Patent(s) as if such Patent Challenge were not underway until the contested Licensed Patent(s) is adjudicated invalid or unenforceable by a court of last resort. If at the end of such Patent Challenge any of the challenged Licensed Patents remain valid, then at Licensor’s option, (i) all royalties and other payments due under this Agreement with respect to such Patent Rights will double or (ii) Licensor may terminate this Agreement if it has not already done so. |
| Section 9 | Term and Termination |
| 9.1 | The term of this license shall begin on the Effective Date of this Agreement and continue until the later of the date that no Licensed Patent remains a pending application or an enforceable patent, or the date on which Licensee’s obligation to pay royalties expires pursuant to Section 4.3 above. |
| 9.2 | Licensee may terminate this Agreement at any time by giving at least sixty (60) days written notice of such termination to Licensor. Such a notice shall be accompanied by a statement of the reasons for termination. |
| 9.3 | Licensor may terminate this Agreement if (a) Licensee (i) is delinquent on any report or payment; (ii) is not diligently developing and commercializing Licensed Products and Licensed Processes; (iii) misses a milestone described in Appendix D; (iv) is in breach of any provision; (v) provides any false report; (vi) goes into bankruptcy, liquidation or proposes having a receiver control any assets; (vii) violates any laws or regulations of applicable government entities; or (viii) shall cease to carry on its business pertaining to Licensed Patents; or (b) if payments of earned royalties under Section 4.3, once begun, cease for more than two (2) calendar quarters. Termination under this Section 9.3 will take effect 30 days after written notice by Licensor, unless Licensee remedies the problem in that 30-day period, except that termination under Section 9.3 (vi) will occur immediately and automatically upon the occurrence of the event and require no action by Licensor. |
| Page 10 |
| 9.4 | If Licensee or any of its Affiliates brings a Patent Challenge against Licensor, or assists another party in bringing a Patent Challenge against Licensor (except as required under a court order or subpoena), then Licensor may immediately terminate this Agreement and/or the license granted hereunder. If a Sublicensee brings a Patent Challenge against Licensor, or assists another party in bringing a Patent Challenge against Licensor (except as required under a court order or subpoena), then Licensor may send a written demand to Licensee to terminate such Sublicense. If Licensee fails to so terminate such Sublicense within forty-five (45) days after Licensor’s demand, Licensor, at its option, may (i) elect to immediately terminate this Agreement and/or the license granted hereunder or (ii) to double all royalties and other payments due under this Agreement with respect to such Patent Rights. |
| 9.5 | If Licensee, any of its Affiliates or a Sublicensee (i) brings a Patent Challenge against Licensor or (ii) assists another party in bringing a Patent Challenge against Licensor (except as required under a court order or subpoena), and if Licensor does not choose to exercise its rights to terminate this Agreement pursuant to Section 9.4 then, in the event that such the Patent Challenge is successful, Licensee will have no right to recoup any consideration, including royalties, paid during the period of challenge. In the event that the Patent Challenge is unsuccessful, Licensee shall reimburse Licensor for all reasonable legal fees and expenses incurred in its defense against the Patent Challenge. |
| 9.6 | Licensor may immediately terminate this Agreement upon the occurrence of the second separate default by Licensee within any consecutive three-year period for failure to pay royalties, patent or any other expenses when due. |
| 9.7 | Upon the termination of this Agreement for any reason, nothing herein shall be construed to release either party from any obligation that matured prior to the effective date of such termination. Licensee shall remain obligated to provide an accounting for and to pay royalties earned to the date of termination, and any minimum royalties shall be prorated as of the date of termination by the number of days elapsed in the applicable calendar year. Licensee may, however, after the effective date of such termination, sell all Licensed Products, and complete Licensed Products in the process of manufacture at the time of such termination and sell the same, provided that Licensee shall remain obligated to provide an accounting for and to pay running royalties thereon. |
| 9.8 | Licensee shall be obligated to deliver to Licensor, within ninety days of the date of termination of this agreement, complete and unredacted copies of all documentation prepared for or submitted for all regulatory approvals of Licensed Products or Licensed Processes. |
| Section 10 | Assignability |
This Agreement may not be transferred or assigned by Licensee except with the prior written consent of Licensor, in which case assignee assumes all responsibilities under this license.
| Page 11 |
| Section 11 | Dispute Resolution Procedures |
| 11.1 | Mandatory Procedures. |
In the event either party intends to file a lawsuit against the other with respect to any matter in connection with this Agreement, compliance with the procedures set forth in this Section shall be a condition precedent to the filing of such lawsuit, other than for injunctive relief. Either party may terminate this Agreement as provided in this Agreement without following the procedures set forth in this Section.
| 11.1.1 | When a party intends to invoke the procedures set forth in this Section, written notice shall be provided to the other party. Within thirty (30) days of the date of such notice, the parties agree that representatives designated by the parties shall meet at mutually agreeable times and engage in good faith negotiations at a mutually convenient location to resolve such dispute. |
| 11.1.2 | If the parties fail to meet within the time period set forth in Section 11.1.1 above or if either party subsequently determines that negotiations between the representatives of the parties are at an impasse, the party declaring that the negotiations are at an impasse shall give notice to the other party stating with particularity the issues that remain in dispute. |
| 11.1.3 | Not more than fifteen (15) days after the giving of such notice of issues, each party shall deliver to the other party a list of the names and addresses of at least three individuals, any one of whom would be acceptable as a neutral advisor in the dispute (the “Neutral Advisor”) to the party delivering the list. Any individual proposed as a Neutral Advisor shall have experience in determining, mediating, evaluating, or trying intellectual property litigation and shall not be affiliated with the party that is proposing such individual. |
| 11.1.4 | Within tend (10) days after delivery of such lists, the parties shall agree on a Neutral Advisor. If they are unable to so agree within that time, within five (5) days, they shall each select one individual from the lists. Within 5 days, the individuals so selected shall meet and appoint a third individual from the lists to serve as the Neutral Advisor. Within thirty (30) days after the selection of a Neutral Advisor: |
| (a) | The parties shall each provide a written statement of the issues in dispute to the Neutral Advisor. |
| (b) | The parties shall meet with the Neutral Advisor in Tampa, Florida on a date and time established by the Neutral Advisor. The meeting must be attended by persons authorized to make final decisions on behalf of each party with respect to the dispute. At the meeting, each party shall make a presentation with respect to its position concerning the dispute. The Neutral Advisor will then discuss the issues separately with each party and attempt to resolve all issues in the dispute. At the meeting, the parties will enter into a written settlement agreement with respect to all issues that are resolved. Such settlement agreement shall be final and binding with respect to such resolved issues and may not be the subject of any lawsuit between the parties, other than a suit for enforcement of the settlement agreement. |
| Page 12 |
| 11.1.5 | The expenses of the neutral advisor shall be shared by the parties equally. All other out- of-pocket costs and expenses for the alternative dispute resolution procedure required under this Section shall be paid by the party incurring the same. |
| 11.1.6 | Positions taken and statements made during this alternative dispute resolution procedure shall be deemed settlement negotiations and shall not be admissible for any purpose in any subsequent proceeding. |
| 11.2 | Failure to Resolve Dispute. |
If any issue is not resolved at the meeting with the Neutral Advisor, either party may file appropriate administrative or judicial proceedings with respect to the issue that remains in dispute. No new issues may be included in the lawsuit without the mandatory procedures set forth in this Section having first been followed.
| Section 12 | Product Liability; Conduct of Business |
| 12.1 | Licensee and its Sublicensee(s) shall, at all times during the term of this Agreement and thereafter, indemnify, defend and hold Licensor, its board, University and its Affiliates and Trustees, the Florida Board of Governors, and each of their directors, officers, employees, and agents, and the inventors of the Licensed Patents, regardless of whether such inventors are employed by Licensor at the time of the claim, harmless against all claims and expenses, including legal expenses and reasonable attorneys fees, whether arising from a third party claim or resulting from Licensor’s enforcing this indemnification clause against Licensee, arising out of the death of or injury to any person or persons or out of any damage to property and against any other claim, proceeding, demand, expense and liability of any kind whatsoever (other than patent infringement claims) resulting from the development, production, manufacture, sale, use, lease, consumption, marketing, or advertisement of Licensed Products or Licensed Process(es) or arising from any right or obligation of Licensee hereunder. Notwithstanding the above, Licensor at all times reserves the right to retain counsel of its own to defend Licensor’s, its board, University and its Affiliates’ and Trustees, the Florida Board of Governors’, and the inventor’s interests. |
| 12.2 | Licensee warrants that it now maintains and will continue to maintain liability insurance coverage appropriate to the risk involved in development, producing, manufacturing, clinical trials, selling, marketing, using, leasing, consuming, or advertising the products subject to this Agreement and that such insurance coverage lists Licensor, its Affiliates, its Trustees, the Florida Board of Governors, and the inventors of the Licensed Patents as additional insureds. Within ninety (90) days after the execution of this Agreement and thereafter annually between January 1 and January 31 of each year, Licensee will present evidence to Licensor that the coverage is being maintained with Licensor, University and its Affiliates and Trustees, the Florida Board of Governors, and its inventors listed as additional insureds. In addition, Licensee shall provide Licensor with at least thirty (30) days prior written notice of any change in or cancellation of the insurance coverage. |
| Page 13 |
| Section 13 | Use of Names |
Licensee and its Sublicensee(s) shall not use the names of Licensor, nor of any of either institution's employees, agents, or affiliates, nor the name of any inventor of Licensed Patents, nor any adaptation of such names, in any promotional, advertising or marketing materials or any other similar form of publicity, or to suggest any endorsement by the such entities or individuals, without the prior written approval of Licensor in each case.
| Section 14 | Miscellaneous |
| 14.1 | This Agreement shall be construed in accordance with the internal laws of the State of Florida without regard to its conflicts of law principles. |
| 14.2 | The parties hereto are independent contractors and not joint venturers or partners. |
| 14.3 | Licensee shall ensure that it applies patent markings that meet all requirements of U.S. law, 35 U.S.C. §287, with respect to all Licensed Products subject to this Agreement. |
| 14.4 | This Agreement constitutes the full understanding between the parties with reference to the subject matter hereof, and no statements or agreements by or between the parties, whether orally or in writing, shall vary or modify the written terms of this Agreement. Neither party shall claim any amendment, modification, or release from any provisions of this Agreement by mutual agreement, acknowledgment, or otherwise, unless such mutual agreement is in writing, signed by the other party, and specifically states that it is an amendment to this Agreement. |
| 14.5 | Licensee shall not encumber or otherwise grant a security interest in any of the rights granted hereunder to any third party. |
| 14.6 | Licensee acknowledges that it is subject to and agrees to abide by the United States laws and regulations (including the Export Administration Act of 1979 and Arms Export Contract Act) controlling the export of technical data, computer software, laboratory prototypes, biological material, and other commodities. The transfer of such items may require a license from the cognizant agency of the U.S. Government or written assurances by Licensee that it shall not export such items to certain foreign countries without prior approval of such agency. Licensor neither represents that a license is or is not required or that, if required, it shall be issued. |
| 14.7 | Licensee is responsible for any and all wire/bank fees associated with all payments due to Licensor pursuant to this agreement. |
| 14.8 | Survival. |
The provisions of this Section shall survive termination of this Agreement. Upon termination of the Agreement for any reason, the following sections of the License Agreement will remain in force as non-cancelable obligations:
| · Section 6 | Record Keeping |
| · Section 9 | Requirement to pay royalties on sale of Licensed Products made, and in process, at time of License Agreement termination |
| · Section 12 | Product Liability; Conduct of Business |
| · Section 13 | Use of Names |
| · Section 18 | Confidentiality |
| Page 14 |
| Section 15 | Notices |
Any notice required to be given pursuant to the provisions of this Agreement shall be in writing and shall be deemed to have been given (a) when delivered personally; or (b) if sent by facsimile transmission, when receipt thereof is acknowledged at the facsimile number of the recipient as set forth below; or (c) the second day following the day on which the notice has been delivered prepaid to a national air courier service; or five (5) business days following deposit in the U.S. mail if sent certified mail, (return receipt acknowledgement is not required to certify delivery).
| 15.1 | All payments and royalty reports to: USF Research Foundation |
Attn: Business Manager
3802 Spectrum Blvd, Suite 100
Tampa, Florida 33612
Development reports; updates; equity agreements, proxy statements and shareholder information; and all other notices and communications to:
USF Technology Transfer Office/Patents & Licensing Attn: Associate Vice President
3802 Spectrum Blvd, Suite 100
Tampa, Florida 33612
| 15.2 | If to Licensee: |
Alzamend Neuro™, Inc.
3802 Spectrum Blvd., Suite 112C Tampa, FL 33612
| Section 16 | Contract Formation and Authority |
The submission of this Agreement does not constitute an offer, and this document shall become effective and binding only upon the execution by duly authorized representatives of both Licensee and Licensor. Copies of this Agreement that have not been executed and delivered by both Licensor and Licensee shall not serve as a memorandum or other writing evidencing an agreement between the parties. This Agreement shall automatically terminate and be of no further force and effect, without the requirement of any notice from Licensor to Licensee, if Licensor does not receive the License Issue Fee or certificates representing shares issued to Licensor pursuant to this Agreement, as applicable, within thirty (30) days of the Effective Date.
| 16.1 | Licensor and Licensee hereby warrant and represent that the persons signing this Agreement have authority to execute this Agreement on behalf of the party for whom they have signed. |
| 16.2 | Force Majeure. |
No default, delay, or failure to perform on the part of Licensee or Licensor shall be considered a default, delay or failure to perform otherwise chargeable hereunder, if such default, delay or failure to perform is due to causes beyond either party’s reasonable control including, but not limited to: strikes, lockouts, or inactions of governmental authorities, epidemics, pandemics, war, embargoes, fire, earthquake, hurricane, flood, acts of God, or default of common carrier. In the event of such default, delay or failure to perform, any date or times by which either party is otherwise scheduled to perform shall be extended automatically for a period of time equal in duration to the time lost by reason of the excused default, delay or failure to perform.
| Page 15 |
| Section 17 | United States Government Interests |
| 17.1 | It is understood that the United States Government (through any of its agencies or otherwise) has funded research during the course of or under which any of the inventions of the Licensed Patents were conceived or made. The United States Government is entitled, as a right, under the provisions of 35 U.S.C. §202-212 and applicable regulations of Title 37 of the Code of Federal Regulations, to a non-exclusive, nontransferable, irrevocable, paid-up license to practice or have practiced the inventions of such Licensed Patents for governmental purposes. Any license granted to Licensee in this Agreement shall be subject to such right. |
| 17.2 | Licensee agrees that for Licensed Products covered by the Licensed Patents that are subject to the non-exclusive royalty-free license to the United States Government, said Licensed Products will be manufactured substantially in the United States. Licensee further agrees that it shall abide by all the requirements and limitations of U.S. Code, Title 35, Chapter 18, and implementing regulations thereof, for all patent applications and patents invented in whole or in part with federal money. |
| Section 18 | Confidentiality |
| 18.1 | Each Party shall maintain all information of the other Party which is treated by such other Party as proprietary or confidential and that is marked “confidential” by the disclosing party or that is confirmed in writing within ten (10) days after verbal disclosure (referred to herein as “Confidential Information”) in confidence, and shall not disclose, divulge or otherwise communicate such confidential information to others, or use it for any purpose, except pursuant to, and in order to carry out, the terms and objectives of this Agreement, and each party hereby agrees to exercise every reasonable precaution to prevent and restrain the unauthorized disclosure of such confidential information by any of its Affiliates, directors, officers, employees, consultants, subcontractors, Sublicensees or agents. The parties agree to keep the terms of this Agreement confidential, provided that each party may disclose this Agreement to their authorized agents and investors who are bound by similar confidentiality provisions. Notwithstanding the foregoing, Confidential Information of a party shall not include information which: (a) was lawfully known by the receiving party prior to disclosure of such information by the disclosing party to the receiving party; (b) was or becomes generally available in the public domain, without the fault of the receiving party; (c) is subsequently disclosed to the receiving party by a third party having a lawful right to make such disclosure; (d) is required by law, rule, regulation or legal process to be disclosed, provided that the receiving party making such disclosure shall take all reasonable steps to restrict and maintain to the extent possible confidentiality of such disclosure and shall provide reasonable notice to the other party to allow such party the opportunity to oppose the required disclosure; or (e) has been independently developed by employees or others on behalf of the receiving party without access to or use of disclosing party’s information as demonstrated by written record. Each party’s obligations under this Section 18 shall extend for a period of five (5) years from termination or expiration of this Agreement. |
| Section 19 | University Rules and Regulations |
| 19.1 | Licensee understands and agrees that Licensor’s personnel who are engaged by Licensee, whether as consultants, employees or otherwise, or who possess a material financial interest in Licensee, are subject to the requirements of the State of Florida and the University regarding outside activities and financial interests, the University’s Intellectual Property Policy and regulations, and a monitoring plan which addresses conflicts of interests associated therewith. Any term or condition of an agreement between Licensee and such personnel which seeks to vary or override such personnel’s obligations to Licensor may not be enforced against such personnel or the Licensor, without the express written consent of an individual authorized to vary or waive such obligations on behalf of the Licensor. Furthermore, should an interest of Licensee conflict with the interest of the Licensor, Licensor’s personnel are obligated to resolve such conflicts according to the guidelines and policies set forth by the Licensor. |
| Page 16 |
IN WITNESS WHEREOF, the parties hereto have duly executed this Agreement on the dates indicated below.
UNIVERSITY OF SOUTH FLORIDA RESEARCH FOUNDATION, INC.
| /s/ David Conrad | Date: June 10, 2020 | |
|
David Conrad, Director Technology Transfer Office |
ALZAMEND NEURO, INC.
| /s/ Stephan Jackman | Date: June 8, 2020 | |
| Stephan Jackman, CEO |
ACKNOWLEDGED AND AGREED:
|
UNIVERSITY OF SOUTH FLORIDA BOARD OF TRUSTEES A PUBLIC BODY CORPORATE |
INVENTOR | ||
| /s/ Keith Anderson | June 10, 2020 | ||
| Keith Anderson, Director | Dr. Roland (Doug) Shytle | ||
| Page 17 |
SCHEDULE 1.8 – LICENSED PATENTS
United States Patent No. 9,840,521, entitled “Organic Anion Lithium Ionic Cocrystal Compounds and Compositions”, filed 09/24/2015 and granted 12/12/2017.
| Page 18 |
Appendix A - Development Plan
| I. | Development Program |
Development activities to be undertaken.
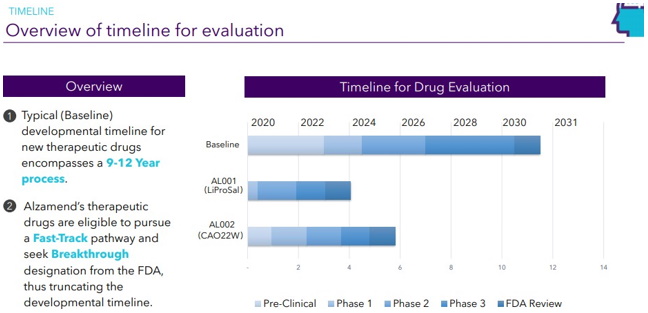
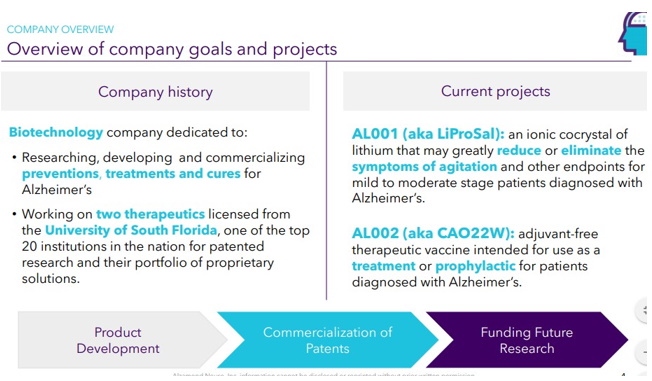
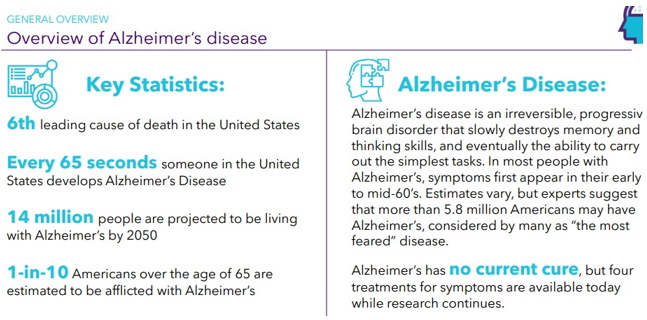
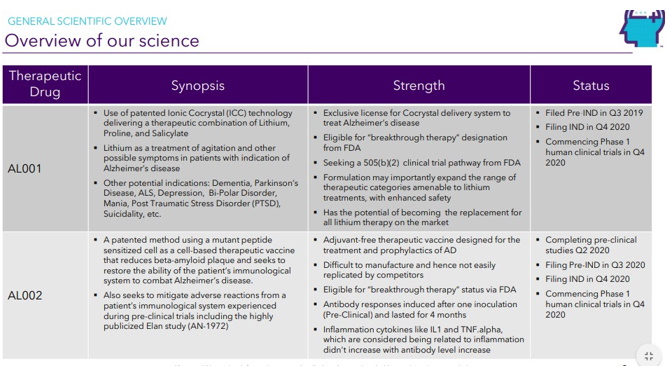
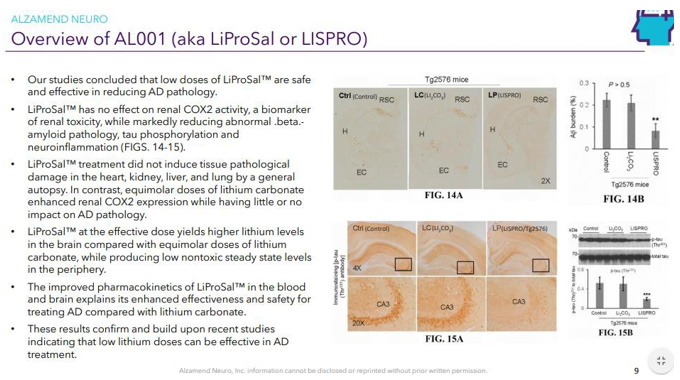
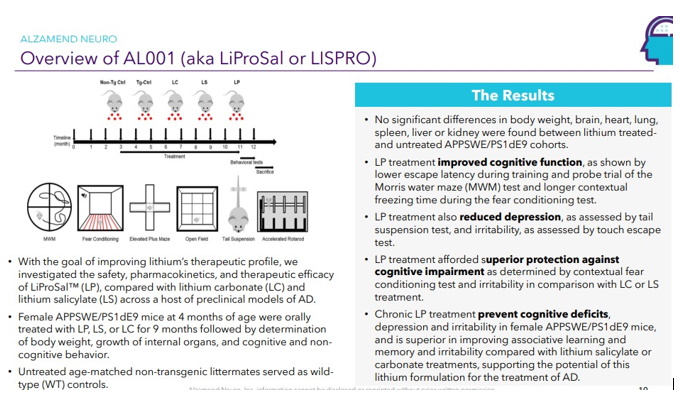
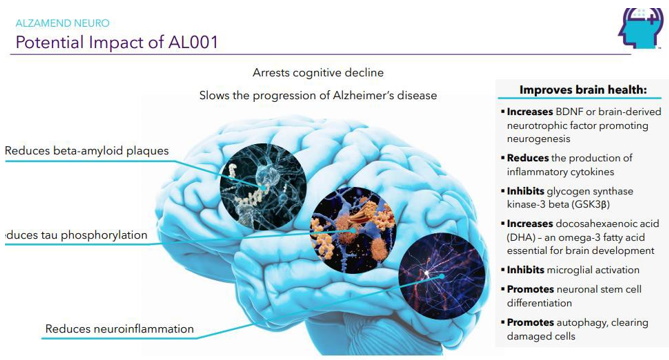
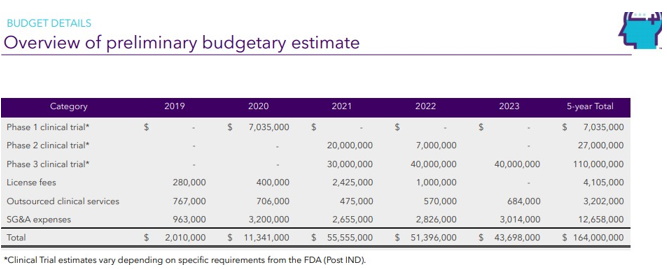
Continue with Reg A+ funding strategy already in place. See attached plan slide deck below that outlines fundraising and preclinical/clinical path:

| Page 19 |
| Page 20 |
| Page 21 |
| Page 22 |

| Page 23 |
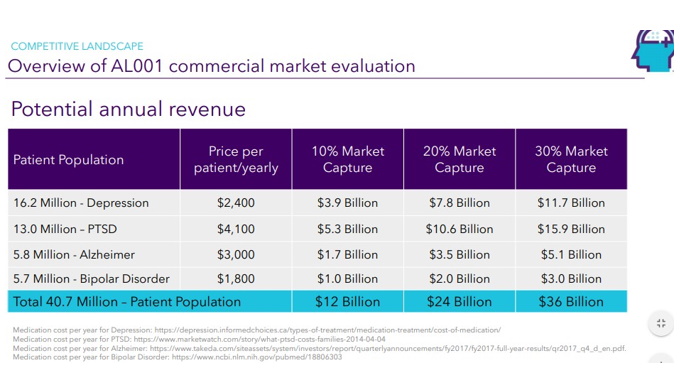

| Page 24 |

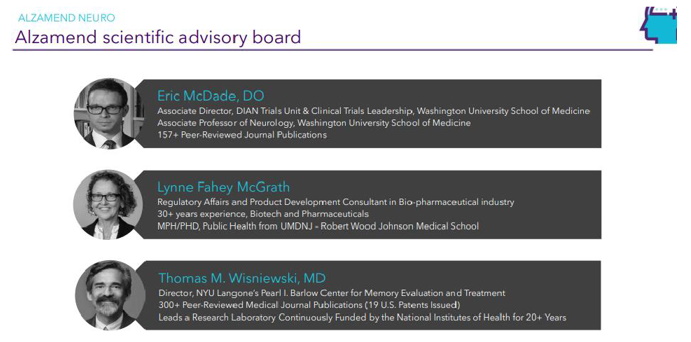
| Page 25 |

| Page 26 |
Appendix B - Development Report
When appropriate, indicate estimated start date and finish date for activities.
| I. | Date Development Plan Initiated and Time Period Covered by this Report. |
| II. | Development Report (4-8 paragraphs). |
| A. | Activities completed since last report including the object and parameters of the development, when initiated, when completed and the results. |
| B. | Activities currently under investigation, i.e., ongoing activities including object and parameters of such activities, when initiated, and projected date of completion. |
| III. | Future Development Activities (4-8 paragraphs). |
| A. | Activities to be undertaken before next report including, but not limited to, the type and object of any studies conducted and their projected starting and completion dates. |
| B. | Estimated total development time remaining before a product will be commercialized. |
| IV. | Changes to Initial Development Plan (2-4 paragraphs). |
| A. | Reasons for change. |
| B. | Variables that may cause additional changes. |
| V. | Items to be Provided if Applicable: |
| A. | Information relating to Licensed Products or Licensed Processes that has become publicly available, e.g., published articles, competing products, patents, etc. |
| B. | Development work being performed by third parties, other than Licensee, to include name of third party, reasons for use of third party, planned future uses of third parties including reasons why and type of work. |
| C. | Update of competitive information trends in industry, government compliance (if applicable) and market plan. |
| D. | Information and copies of relevant materials evidencing the status of any patent applications or other protection relating to Licensed Products, or Licensed Processes or the Licensed Patents. |
PLEASE SEND DEVELOPMENT REPORTS TO:
USF Division of Patents & Licensing
Attn: Associate Vice President
3802 Spectrum Blvd, Suite 100
Tampa, Florida 33612
| Page 1 | Initials ________________ |
Appendix C - Licensor Royalty Report
| Licensee: | |||
| Agreement No.: | |||
| Inventor: | |||
| Technology#: | |||
| Period Covered: | From: / /2 | Through: / /2 | |
| Prepared By: | |||
| Date: | |||
| Approved By: | |||
| Date: | |||
If license covers several major product lines, please prepare a separate report for each line. Then combine all product lines into a summary report.
| Report Type: ¨ Single Product Line Report: |
| ¨ Multiproduct Summary Report. Page 1 of ______ Pages | |
| ¨ Product Line Detail. Line: Tradename: Page: _______________ |
| Report Currency: | ¨ U. S. Dollars | ¨ Other |
| Unit | Gross | * Less: | Net | Royalty | Period Royalty Amount | ||
| Country | Sales | $$ Sales | Allowances | $$ Sales | Rate | This Year | Last Year |
| U.S.A. | |||||||
| Canada | |||||||
| Europe: | |||||||
| Japan | |||||||
| Other: | |||||||
| TOTAL: | |||||||
| Total Royalty: | Conversion Rate: | Royalty in U.S. Dollars: | $ |
The following royalty forecast is non-binding and for Licensor’s internal planning purposes only:
Royalty Forecast Under This Agreement:
| Next Quarter: | Q2: | Q3: | Q4: |
| Page 1 | Initials ________________ |
| Total Royalty: | Conversion Rate: | Royalty in U.S. Dollars: | $ |
The following royalty forecast is non-binding and for Licensor’s internal planning purposes only:
| Royalty Forecast Under This Agreement: | Next Quarter: | Q2: | Q3: | Q4: |
| * On a separate page, please indicate the reasons for returns or other adjustments if significant. |
| Also note any unusual occurrences that affected royalty amounts during this period. |
| To assist Licensor’s forecasting, please comment on any significant expected trends in sales volume. |
PLEASE SEND ROYALTY REPORTS TO:
USF Research Foundation
Attn: Business Manager
3802 Spectrum Blvd, Suite 100
Tampa, Florida 33612
| Page 2 | Initials ________________ |
Appendix D - Milestones
| 1. | Licensee has already provided Licensor a complete business plan and detailed management team attached herein as Appendix A. |
| 2. | By 20 months from the Effective Date, Licensee will have $5,000,000 of available non-contingent, operating capital to proceed with the exploration and development of Licensed Product. Capital will be from a third party who may or may not be an investor in Licensee and unused capital will be on deposit in a financial institutional acceptable to both Licensor and Licensee. |
| 3. | Company will meet the following Regulatory Milestones: |
| Due Date | Event | ||
| Completed September 2019 | Pre-IND Meeting | ||
| October 30, 2020 | IND filing | ||
| 12 months from IND filing | First dosing of a patient in a clinical trial | ||
| 12 months from completion of the first dosing of a patient | Completion of first clinical trial | ||
| 36 months from completion of the first Phase II Clinical Trial | First patient treated in a Phase III Clinical Trial | ||
| July 1, 2027 | First Commercial Sale |
Page 0 of 1