
Exhibit 1U-15.2

TM Shareholder & Investor Conference Call & Webcast November 17, 2020

SAFE HARBOR STATEMENT 2 This presentation contains forward - looking statements. All statements other than statements of historical fact are, or may be de emed to be, forward - looking statements. Such forward - looking statements include statements regarding, among others, (a) our expectations about possible business combina tions, (b) our growth strategies, (c) our future financing plans, and (d) our anticipated needs for working capital. Forward - looking statements, which involve assumpt ions and describe our future plans, strategies, and expectations, are generally identifiable by use of the words “may,” “will,” “should,” “expect,” “anticipate,” “a pproximate,” “estimate,” “believe,” “intend,” “plan,” “budget,” “could,” “forecast,” “might,” “predict,” “shall” or “project,” or the negative of these words or other vari ati ons on these words or comparable terminology. This information may involve known and unknown risks, uncertainties, and other factors that may cause our actual re sults, performance, or achievements to be materially different from the future results, performance, or achievements expressed or implied by any forward - looking statem ents. These statements may be found in the Annual Report and in the Semiannual Report referred to immediately below. This presentation should be read in conjunction with the audited financial statements and related notes for the fiscal year e nde d April 30, 2019, contained in the Company’s Annual Report on Form 1 - K, as filed with the Securities and Exchange Commission on August 28, 2019. Forward - looking statements are based on our current expectations and assumptions regarding our business, potential target busine sses, the economy and other future conditions. Because forward - looking statements relate to the future, by their nature, they are subject to inherent uncertainties , risks, and changes in circumstances that are difficult to predict. Our actual results may differ materially from those contemplated by the forward - looking statements as a result of various factors, including, without limitation, changes in local, regional, national or global political, economic, business, competitive, market (supply an d demand) and regulatory conditions and the following: • Our ability to effectively execute our business plan; • Our ability to manage our expansion, growth and operating expenses; • Our ability to evaluate and measure our business, prospects and performance metrics; • Our ability to compete and succeed in a highly competitive and evolving industry; • Our ability to respond and adapt to changes in technology and customer behavior; and • Our ability to protect our intellectual property and to develop, maintain and enhance a strong brand. We caution you therefore that you should not rely on any of these forward - looking statements as statements of historical fact or as guarantees or assurances of future performance. All forward - looking statements speak only as of the date of this presentation. We undertake no obligation to update any forward - looking statements or other information contained herein. Information regarding market and industry statistics contained in this presentation is included based on information availabl e t o us that we believe is accurate. It is generally based on academic and other publications that are not produced for purposes of securities offerings or economic ana lys is. Forecasts and other forward - looking information obtained from these sources are subject to the same qualifications and the additional uncertainties accom pan ying any estimates of future market size, revenue and market acceptance of products and services. Except as required by U.S. federal securities laws, we have no obl igation to update forward - looking information to reflect actual results or changes in assumptions or other factors that could affect those statements.
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. TABLE OF CONTENTS 3 Introduction Company Overview 4 Our Science General Scientific Overview 5 Specific Details Budget Details 6 Final Details Capital Raise 7 Our Team 8 - 12
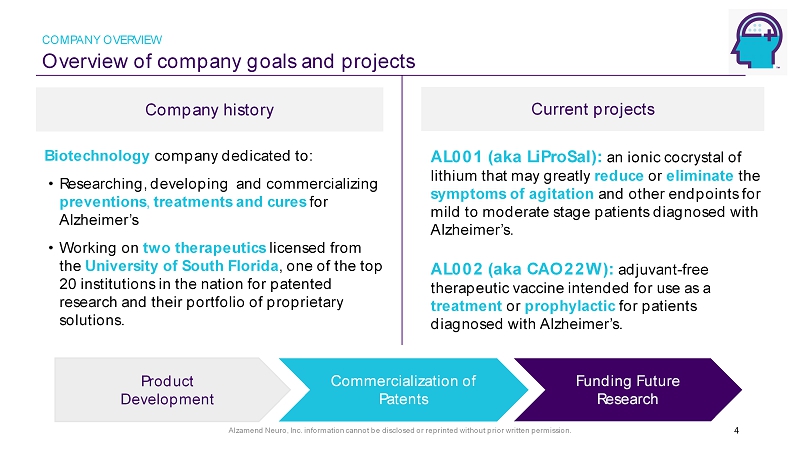
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of company goals and projects COMPANY OVERVIEW Company history Current projects 4 AL001 (aka LiProSal): an ionic cocrystal of lithium that may greatly reduce or eliminate the symptoms of agitation and other endpoints for mild to moderate stage patients diagnosed with Alzheimer’s. AL002 (aka CAO22W): adjuvant - free therapeutic vaccine intended for use as a treatment or prophylactic for patients diagnosed with Alzheimer’s. Biotechnology company dedicated to: • Researching, developing and commercializing preventions , treatments and cures for Alzheimer’s • Working on two therapeutics licensed from the University of South Florida , one of the top 20 institutions in the nation for patented research and their portfolio of proprietary solutions. Commercialization of Patents Funding Future Research Product Development
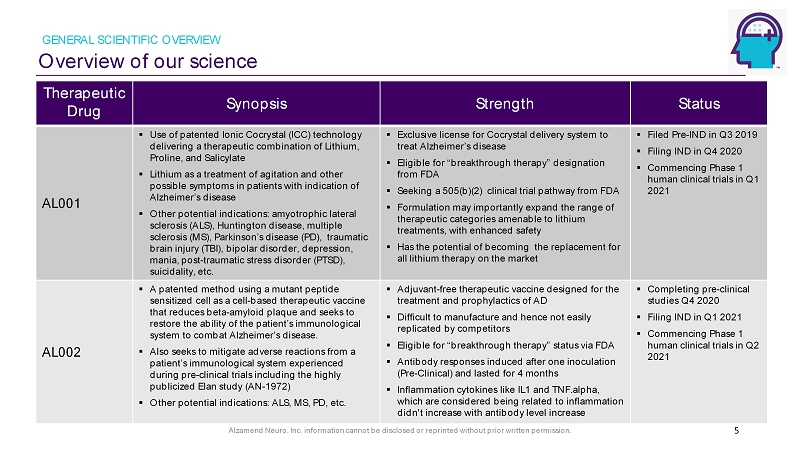
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of our science GENERAL SCIENTIFIC OVERVIEW 5 Therapeutic Drug Synopsis Strength Status AL001 ▪ Use of patented Ionic Cocrystal (ICC) technology delivering a therapeutic combination of Lithium, Proline, and Salicylate ▪ Lithium as a treatment of agitation and other possible symptoms in patients with indication of Alzheimer’s disease ▪ Other potential indications: amyotrophic lateral sclerosis (ALS), Huntington disease, multiple sclerosis (MS), Parkinson’s disease (PD ), traumatic brain injury (TBI), bipolar disorder, depression, mania, post - traumatic stress disorder (PTSD), suicidality, etc. ▪ Exclusive license for Cocrystal delivery system to treat Alzheimer’s disease ▪ Eligible for “breakthrough therapy” designation from FDA ▪ Seeking a 505(b)(2) clinical trial pathway from FDA ▪ Formulation may importantly expand the range of therapeutic categories amenable to lithium treatments, with enhanced safety ▪ Has the potential of becoming the replacement for all lithium therapy on the market ▪ Filed Pre - IND in Q3 2019 ▪ Filing IND in Q4 2020 ▪ Commencing Phase 1 human clinical trials in Q1 2021 AL002 ▪ A patented method using a mutant peptide sensitized cell as a cell - based therapeutic vaccine that reduces beta - amyloid plaque and seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s disease. ▪ Also seeks to mitigate adverse reactions from a patient’s immunological system experienced during pre - clinical trials including the highly publicized Elan study (AN - 1972) ▪ Other potential indications: ALS, MS, PD, etc. ▪ Adjuvant - free therapeutic vaccine designed for the treatment and prophylactics of AD ▪ Difficult to manufacture and hence not easily replicated by competitors ▪ Eligible for “breakthrough therapy” status via FDA ▪ Antibody responses induced after one inoculation (Pre - Clinical) and lasted for 4 months ▪ Inflammation cytokines like IL1 and TNF.alpha, which are considered being related to inflammation didn't increase with antibody level increase ▪ Completing pre - clinical studies Q4 2020 ▪ Filing IND in Q1 2021 ▪ Commencing Phase 1 human clinical trials in Q2 2021
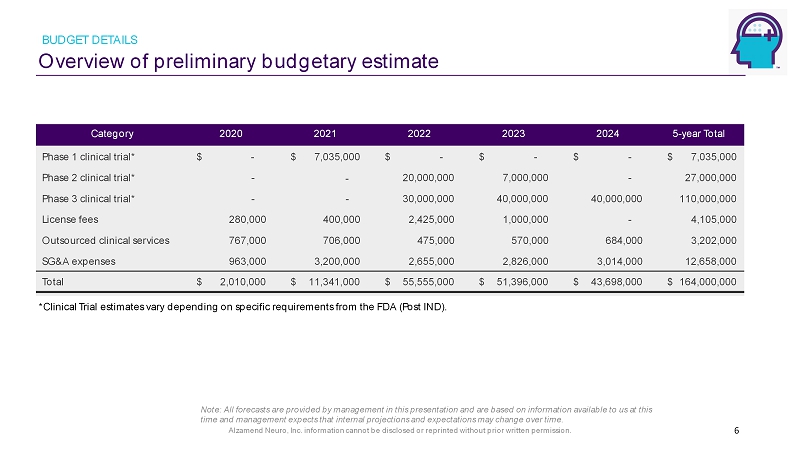
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of preliminary budgetary estimate BUDGET DETAILS 6 Category 2020 2021 2022 2023 2024 5 - year Total Phase 1 clinical trial* $ 7,035,000 $ - $ - $ - $ 7 ,035,000 $ Phase 2 clinical trial* - 20 ,000,000 7,000,000 - 27,000,000 Phase 3 clinical trial* - - 30,000,000 40,000,000 40,000,000 110,000,000 License fees 280,000 400,000 2,425,000 1,000,000 - 4,105,000 Outsourced clinical services 767,000 70 6,000 475,000 570,000 684,000 3,202,000 SG&A expenses 963,000 3 ,200,000 2,655,000 2,826,000 3,014,000 12,658,000 Total 2 ,010,000 $ 1 1 , 341 ,000 $ 55 ,555,000 $ 51,396,000 $ 43,698,000 $ 164,000,000 $ - - *Clinical Trial estimates vary depending on specific requirements from the FDA (Post IND). Note: All forecasts are provided by management in this presentation and are based on information available to us at this time and management expects that internal projections and expectations may change over time.
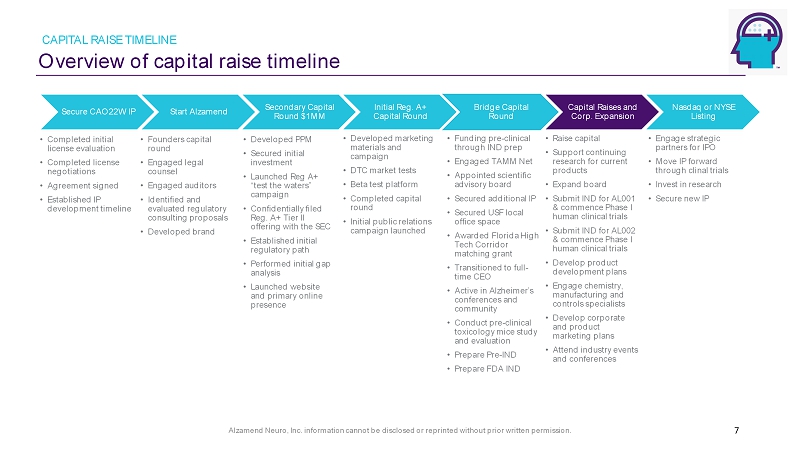
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of capital raise timeline CAPITAL RAISE TIMELINE 7 Secure CAO22W IP • Completed initial license evaluation • Completed license negotiations • Agreement signed • Established IP development timeline Start Alzamend • Founders capital round • Engaged legal counsel • Engaged auditors • Identified and evaluated regulatory consulting proposals • Developed brand Secondary Capital Round $1MM • Developed PPM • Secured initial investment • Launched Reg A+ “test the waters” campaign • Confidentially filed Reg. A+ Tier II offering with the SEC • Established initial regulatory path • Performed initial gap analysis • Launched website and primary online presence Initial Reg. A+ Capital Round • Developed marketing materials and campaign • DTC market tests • Beta test platform • Completed capital round • Initial public relations campaign launched Bridge Capital Round • Funding pre - clinical through IND prep • Engaged TAMM Net • Appointed scientific advisory board • Secured additional IP • Secured USF local office space • Awarded Florida High Tech Corridor matching grant • Transitioned to full - time CEO • Active in Alzheimer’s conferences and community • Conduct pre - clinical toxicology mice study and evaluation • Prepare Pre - IND • Prepare FDA IND Capital Raises and Corp. Expansion • Raise capital • Support continuing research for current products • Expand board • Submit IND for AL001 & commence Phase I human clinical trials • Submit IND for AL002 & commence Phase I human clinical trials • Develop product development plans • Engage chemistry, manufacturing and controls specialists • Develop corporate and product marketing plans • Attend industry events and conferences Nasdaq or NYSE Listing • Engage strategic partners for IPO • Move IP forward through clinal trials • Invest in research • Secure new IP

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend leadership team ALZAMEND NEURO 8 Stephan Jackman Chief Executive Officer 20+ years multi - industry experience, specialized in Biotech and Pharmaceutical David Katzoff SVP Operations 30+ Years multi - industry experience, including Healthcare and Technology Kenneth S. Cragun Chief Financial Officer 30+ Years SEC reporting, Nasdaq CFO, multi - industry experience, including Biotech and Healthcare Henry Nisser EVP, General Counsel and Corporate Secretary 20+ years experience, U.S. securities compliance, M&A, equity/debt financings and corporate governance

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend board of directors ALZAMEND NEURO 9 Milton “Todd” Ault, III Founder/Executive Chairman of Alzamend Chairman & CEO of DPW Holdings 27+ years Financial Industry experience, seasoned Wall Street CEO & activist investor William B. Horne Chief Financial Officer at DPW Holdings 25+ years Financial Industry experience, prior “Big 4” auditor & healthcare executive Philip E. Mansour CEO at MTIX International 25+ years multi - industry experience, seasoned executive, manager & coach Henry Nisser EVP, General Counsel and Corporate Secretary 20+ years experience, U.S. securities compliance, M&A, equity/debt financings and corporate governance Stephan Jackman Chief Executive Officer 20+ years multi - industry experience, specialized in Biotech and Pharmaceutical

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend scientific advisory board ALZAMEND NEURO 10 Eric McDade , DO Associate Director, DIAN Trials Unit & Clinical Trials Leadership, Washington University School of Medicine Associate Professor of Neurology, Washington University School of Medicine 157+ Peer - Reviewed Journal Publications Thomas M. Wisniewski, MD Director, NYU Langone’s Pearl I. Barlow Center for Memory Evaluation and Treatment 300+ Peer - Reviewed Medical Journal Publications (19 U.S. Patents Issued) Leads a Research Laboratory Continuously Funded by the National Institutes of Health for 20+ Years

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend scientists/inventors ALZAMEND NEURO 11 Roland Shytle , PhD. Co - Inventor of AL001 (LiProSal) Associate Professor, Center of Excellence for Aging & Brain Repair, University of South Florida 30+ Peer - Reviewed Journal Publications (2 U.S. Patents Issued) 30+ Years experience and a leading researcher in Allergy, Immunology and Neurodegenerative Disease Chuanhai Cao, PhD. Inventor of AL002 (CAO22W) Assistant Professor, College of Medicine Neurology, University of South Florida 70+ Peer - Reviewed Journal Publications (4 U.S. Patents Issued) 30+ Years experience and a leading researcher in the field of Alzheimer's treatments
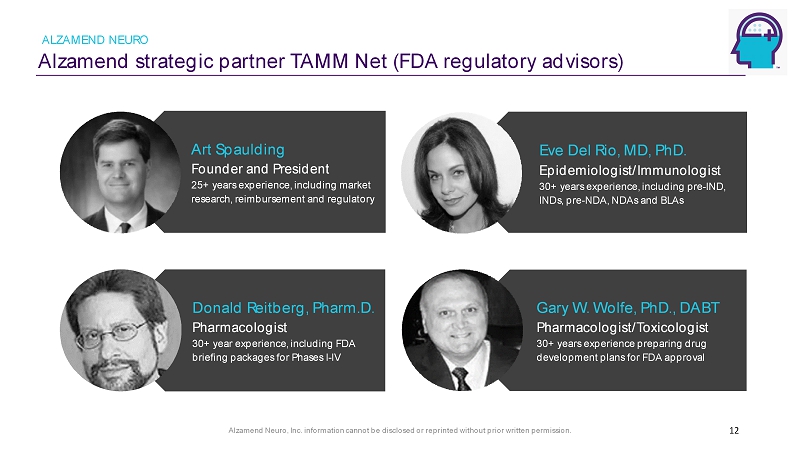
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend strategic partner TAMM Net (FDA regulatory advisors) ALZAMEND NEURO 12 Art Spaulding Founder and President 25+ years experience, including market research, reimbursement and regulatory Donald Reitberg , Pharm.D. Pharmacologist 30+ year experience, including FDA briefing packages for Phases I - IV Eve Del Rio, MD, PhD. Epidemiologist/Immunologist 30+ years experience, including pre - IND, INDs, pre - NDA, NDAs and BLAs Gary W. Wolfe, PhD., DABT Pharmacologist/Toxicologist 30+ years experience preparing drug development plans for FDA approval

13 Thank You! November 2020