
Exhibit 99.1

Alzamend Neuro, Inc. Corporate Presentation April 2022

SAFE HARBOR STATEMENT 2 This presentation and other written or oral statements made from time to time by representatives of Alzamend Neuro, Inc . (the “Company” or “ Alzamend ”) contain “forward looking statements” within the meaning of Section 27 A of the Securities Act of 1933 , as amended, and Section 21 E of the Securities Exchange Act of 1934 , as amended . Forward - looking statements reflect the current view about future events . Statements that are not historical in nature, such as forecasts for the industry in which we operate, and which may be identified by the use of words like “ expects,” “assumes,” “projects,” “anticipates,” “estimates,” “we believe,” “could be,” “future,”" or the negative of these terms and other words of similar meaning, are forward - looking statements . Such statements include, but are not limited to, statements contained in this presentation relating to our business, business strategy, expansion, growth and product candidates and the timing of their development, sales and marketing strategy and capital outlook . Forward - looking statements are based on management’s current expectations and assumptions regarding our business, the economy and other future conditions and are subject to inherent risks, uncertainties and changes of circumstances that are difficult to predict and may cause actual results to differ materially from those contemplated or expressed . We caution you therefore against relying on any of these forward - looking statements . These risks and uncertainties include those risk factors discussed in Part I, “Item 1 A . Risk Factors” of our Annual Report on Form 10 - K for the fiscal year ended April 30 , 2020 (the “ 2020 Annual Report”) and other information contained in subsequently filed current and periodic reports, each of which is available on our website and on the Securities and Exchange Commission’s website ( www . sec . gov ) . Any forward - looking statements are qualified in their entirety by reference to the risk factors discussed in the 2020 Annual Report . Should one or more of these risks or uncertainties materialize (or in certain cases fail to materialize), or should the underlying assumptions prove incorrect, actual results may differ significantly from those anticipated, believed, estimated, expected, intended or planned . Important factors that could cause actual results to differ materially from those in the forward - looking statements include : risks related to performing clinical studies ; the ability to initiate and complete clinical studies and report data therefrom ; whether the results from clinical studies will validate and support the safety and efficacy of our product candidates ; competition from other products ; risks in product development ; the ability to protect our intellectual property rights ; impact of any litigation or infringement actions brought against us ; market acceptance if we can commercialize our product candidates ; inability to raise capital to fund clinical trials ; and changes in government regulation . Factors or events that could cause our actual results to differ may emerge from time to time, and it is not possible for us to predict all of them . We cannot guarantee future results, levels of activity, performance or achievements . Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward - looking statements to conform these statements to actual results . All forecasts are provided by management in this presentation and are based on information available to us at this time and management expects that internal projections and expectations may change over time . In addition, the forecasts are based entirely on management’s best estimate of our future financial performance given our product candidate development and market opportunities .
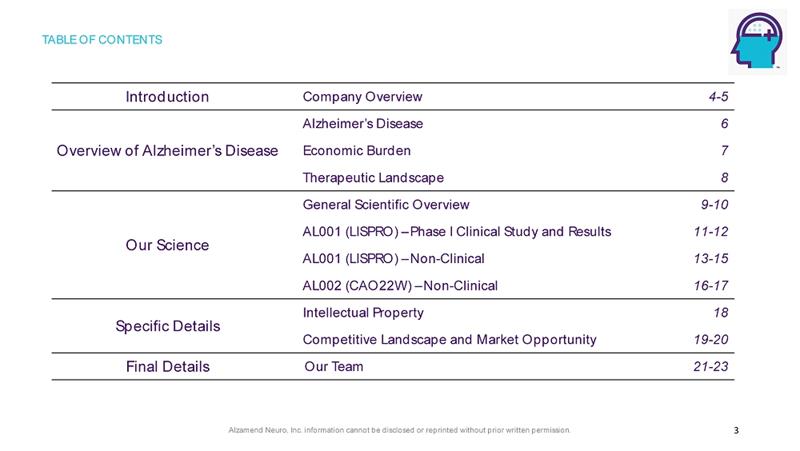
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. TABLE OF CONTENTS 3 Introduction Company Overview 4 - 5 Overview of Alzheimer’s Disease Alzheimer’s Disease 6 Economic Burden 7 Therapeutic Landscape 8 Our Science General Scientific Overview 9 - 10 AL001 (LISPRO) – Phase I Clinical Study and Results 11 - 12 AL001 (LISPRO) – Non - Clinical 13 - 15 AL002 (CAO22W) – Non - Clinical 16 - 17 Specific Details Intellectual Property 18 Competitive Landscape and Market Opportunity 19 - 20 Final Details Our Team 21 - 23
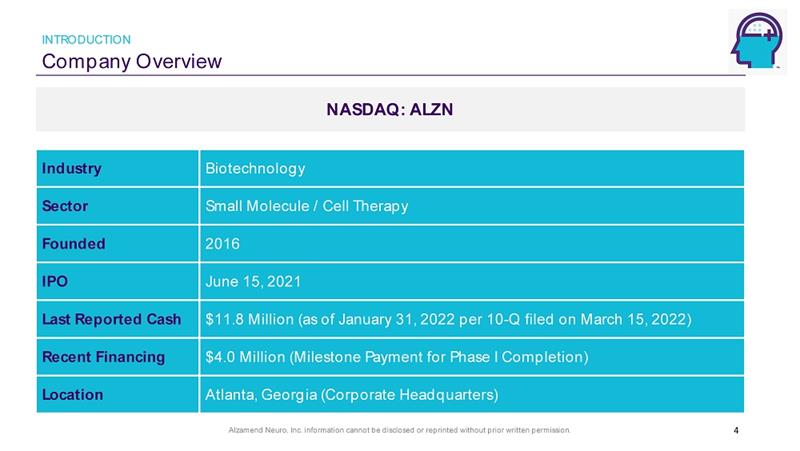
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Company Overview INTRODUCTION NASDAQ: ALZN 4 Industry Biotechnology Sector Small Molecule / Cell Therapy Founded 2016 IPO June 15, 2021 Last Reported Cash $11.8 Million (as of January 31, 2022 per 10 - Q filed on March 15, 2022) Recent Financing $4.0 Million (Milestone Payment for Phase I Completion) Location Atlanta, Georgia (Corporate Headquarters)
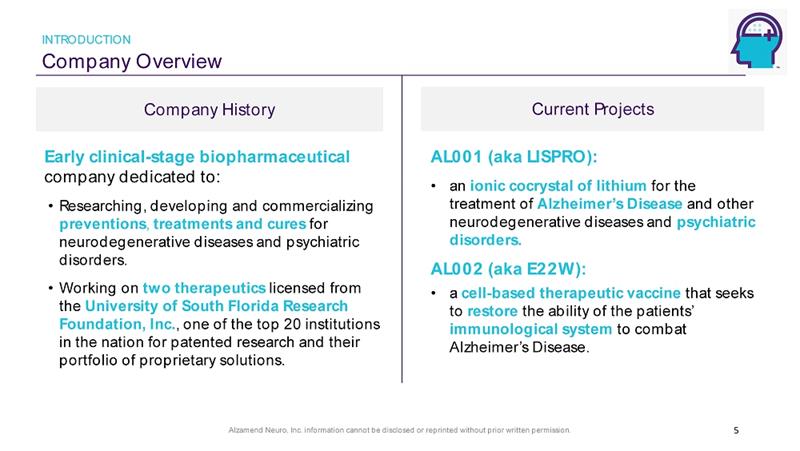
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Company Overview INTRODUCTION Company History Current Projects 5 AL001 (aka LISPRO): • an ionic cocrystal of lithium for the treatment of Alzheimer’s Disease and other neurodegenerative diseases and psychiatric disorders. AL002 (aka E22W): • a cell - based therapeutic vaccine that seeks to restore the ability of the patients’ immunological system to combat Alzheimer’s Disease. Early clinical - stage biopharmaceutical company dedicated to: • Researching, developing and commercializing preventions , treatments and cures for neurodegenerative diseases and psychiatric disorders. • Working on two therapeutics licensed from the University of South Florida Research Foundation, Inc. , one of the top 20 institutions in the nation for patented research and their portfolio of proprietary solutions.
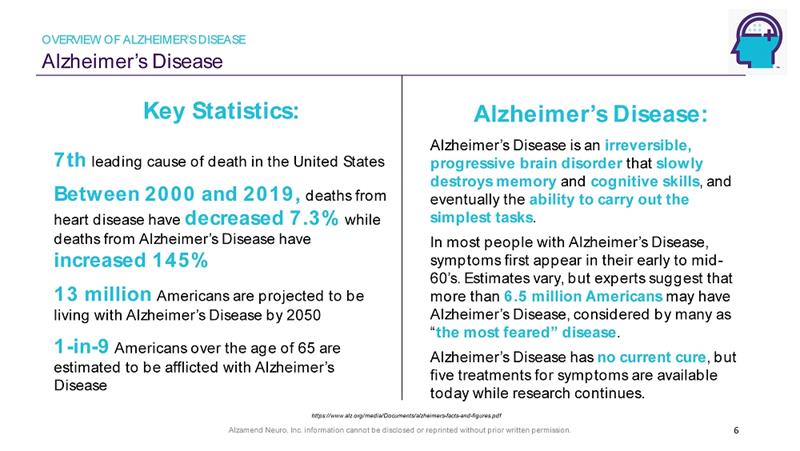
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzheimer’s Disease OVERVIEW OF ALZHEIMER’S DISEASE 6 Alzheimer’s Disease: Alzheimer’s Disease is an irreversible, progressive brain disorder that slowly destroys memory and cognitive skills , and eventually the ability to carry out the simplest tasks . In most people with Alzheimer’s Disease, symptoms first appear in their early to mid - 60’s. Estimates vary, but experts suggest that more than 6.5 million Americans may have Alzheimer’s Disease, considered by many as “ the most feared” disease . Alzheimer’s Disease has no current cure , but five treatments for symptoms are available today while research continues. Key Statistics: 7th leading cause of death in the United States Between 2000 and 2019, deaths from heart disease have decreased 7.3% while deaths from Alzheimer’s Disease have increased 145% 13 million Americans are projected to be living with Alzheimer’s Disease by 2050 1 - in - 9 Americans over the age of 65 are estimated to be afflicted with Alzheimer’s Disease https://www.alz.org/media/Documents/alzheimers - facts - and - figures.pdf
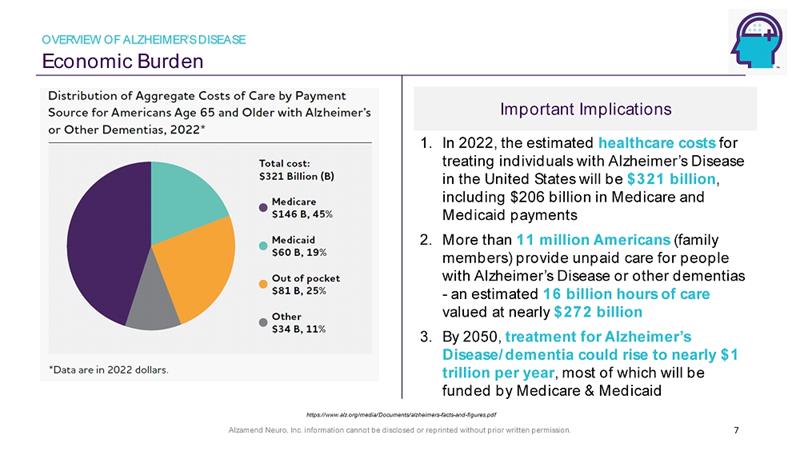
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Important Implications Economic Burden 1. In 2022, the estimated healthcare costs for treating individuals with Alzheimer’s Disease in the United States will be $321 billion , including $206 billion in Medicare and Medicaid payments 2. More than 11 million Americans (family members) provide unpaid care for people with Alzheimer’s Disease or other dementias - an estimated 16 billion hours of care valued at nearly $272 billion 3. By 2050, treatment for Alzheimer’s Disease/dementia could rise to nearly $1 trillion per year , most of which will be funded by Medicare & Medicaid 7 https://www.alz.org/media/Documents/alzheimers - facts - and - figures.pdf OVERVIEW OF ALZHEIMER’S DISEASE
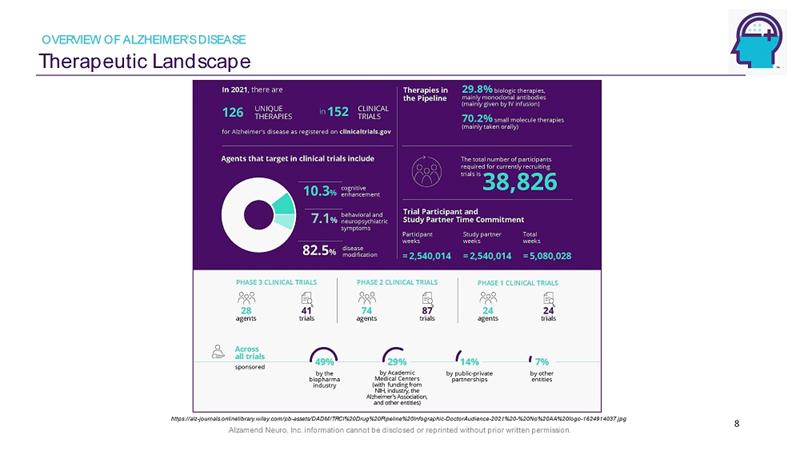
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Therapeutic Landscape 8 OVERVIEW OF ALZHEIMER’S DISEASE https://alz - journals.onlinelibrary.wiley.com/pb - assets/DADM/TRCI%20Drug%20Pipeline%20Infographic - DoctorAudience - 2021%20 - %20No%20 AA%20logo - 1624914037.jpg
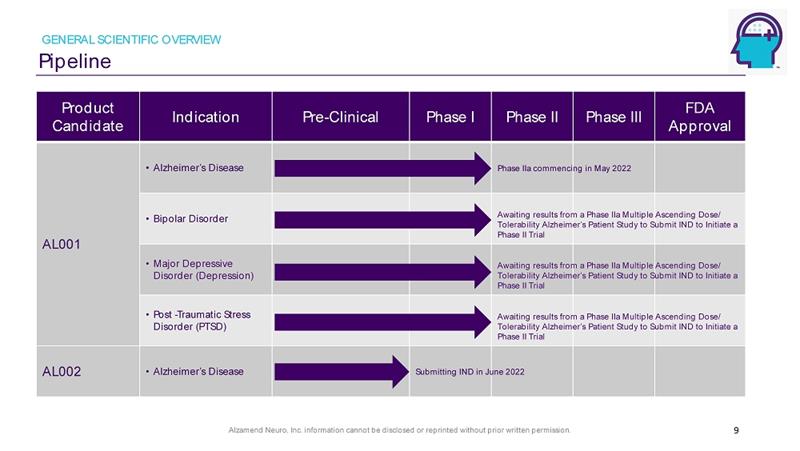
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Pipeline GENERAL SCIENTIFIC OVERVIEW 9 Product Candidate Indication Pre - Clinical Phase I Phase II Phase III FDA Approval AL001 • Alzheimer’s Disease • Bipolar Disorder • Major Depressive Disorder (Depression) • Post - Traumatic Stress Disorder (PTSD) AL002 • Alzheimer’s Disease Awaiting results from a Phase IIa Multiple Ascending Dose/ Tolerability Alzheimer’s Patient Study to Submit IND to Initiate a Phase II Trial Phase IIa commencing in May 2022 Awaiting results from a Phase IIa Multiple Ascending Dose/ Tolerability Alzheimer’s Patient Study to Submit IND to Initiate a Phase II Trial Awaiting results from a Phase IIa Multiple Ascending Dose/ Tolerability Alzheimer’s Patient Study to Submit IND to Initiate a Phase II Trial Submitting IND in June 2022
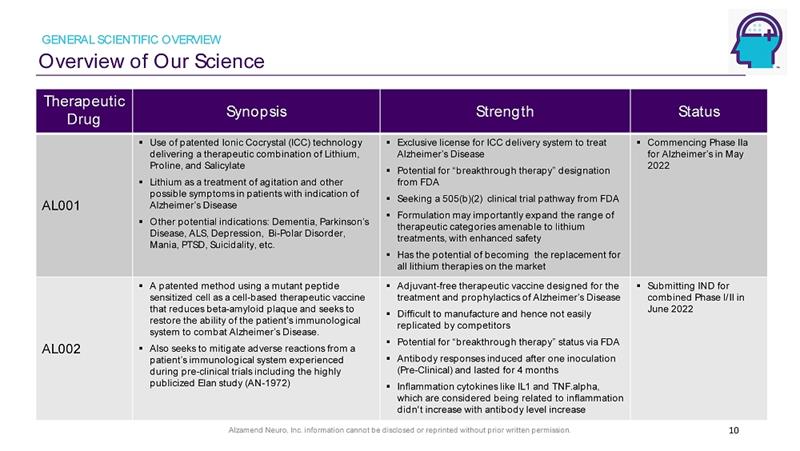
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of Our Science GENERAL SCIENTIFIC OVERVIEW 10 Therapeutic Drug Synopsis Strength Status AL001 ▪ Use of patented Ionic Cocrystal (ICC) technology delivering a therapeutic combination of Lithium, Proline, and Salicylate ▪ Lithium as a treatment of agitation and other possible symptoms in patients with indication of Alzheimer’s Disease ▪ Other potential indications: Dementia, Parkinson’s Disease, ALS, Depression, Bi - Polar Disorder, Mania, PTSD, Suicidality, etc. ▪ Exclusive license for ICC delivery system to treat Alzheimer’s Disease ▪ Potential for “breakthrough therapy” designation from FDA ▪ Seeking a 505(b)(2) clinical trial pathway from FDA ▪ Formulation may importantly expand the range of therapeutic categories amenable to lithium treatments, with enhanced safety ▪ Has the potential of becoming the replacement for all lithium therapies on the market ▪ Commencing Phase IIa for Alzheimer’s in May 2022 AL002 ▪ A patented method using a mutant peptide sensitized cell as a cell - based therapeutic vaccine that reduces beta - amyloid plaque and seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s Disease. ▪ Also seeks to mitigate adverse reactions from a patient’s immunological system experienced during pre - clinical trials including the highly publicized Elan study (AN - 1972) ▪ Adjuvant - free therapeutic vaccine designed for the treatment and prophylactics of Alzheimer’s Disease ▪ Difficult to manufacture and hence not easily replicated by competitors ▪ Potential for “breakthrough therapy” status via FDA ▪ Antibody responses induced after one inoculation (Pre - Clinical) and lasted for 4 months ▪ Inflammation cytokines like IL1 and TNF.alpha, which are considered being related to inflammation didn't increase with antibody level increase ▪ Submitting IND for combined Phase I/II in June 2022
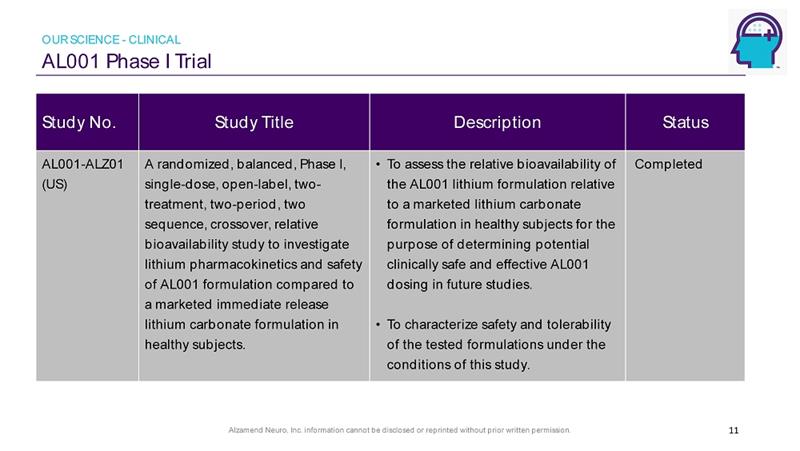
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. AL001 Phase I Trial 11 Study No. Study Title Description Status AL001 - ALZ01 (US) A randomized, balanced, Phase I, single - dose, open - label, two - treatment, two - period, two sequence, crossover, relative bioavailability study to investigate lithium pharmacokinetics and safety of AL001 formulation compared to a marketed immediate release lithium carbonate formulation in healthy subjects. • To assess the relative bioavailability of the AL001 lithium formulation relative to a marketed lithium carbonate formulation in healthy subjects for the purpose of determining potential clinically safe and effective AL001 dosing in future studies. • To characterize safety and tolerability of the tested formulations under the conditions of this study. Completed OUR SCIENCE - CLINICAL

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Safety/Tolerability: Primary Endpoint Met Select Results from Phase I Trial 12 » AL001 was shown to be safe and well - tolerated in healthy adult subjects » No serious adverse events and no deaths were reported during the trial » T he safety profiles of both AL001 and the marketed lithium carbonate capsule were benign » No clinically significant abnormal findings in electrocardiograms were noted during the trial » AL001 salicylate plasma concentrations were observed to be well tolerated and consistently within safe limits » Dose - adjusted relative bioavailability analyses of the rate and extent of lithium absorption in plasma indicated that AL001 1050 mg (lithium content equivalent to150 mg lithium carbonate) is bioequivalent to a marketed 300 mg lithium carbonate capsule and the shapes of the lithium plasma concentration versus time curves are similar OUR SCIENCE - CLINICAL
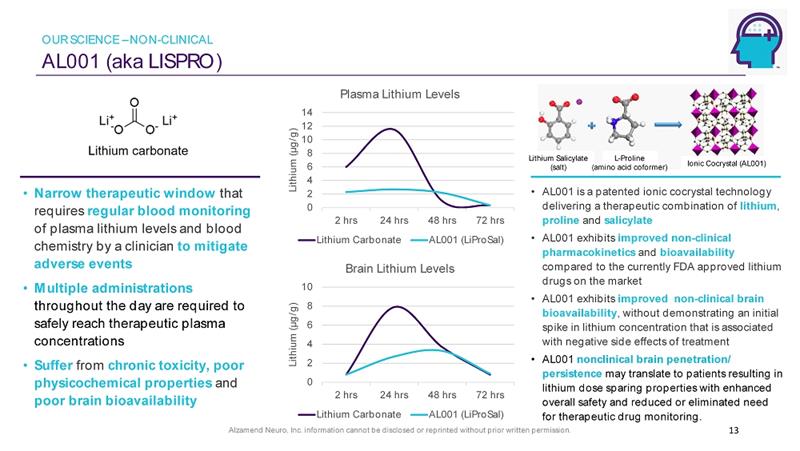
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. AL001 (aka LISPRO ) 13 • AL001 is a patented ionic cocrystal technology delivering a therapeutic combination of lithium , proline and salicylate • AL001 exhibits improved non - clinical pharmacokinetics and bioavailability compared to the currently FDA approved lithium drugs on the market • AL001 exhibits improved non - clinical brain bioavailability , without demonstrating an initial spike in lithium concentration that is associated with negative side effects of treatment • AL001 nonclinical brain penetration/ persistence may translate to patients resulting in lithium dose sparing properties with enhanced overall safety and reduced or eliminated need for therapeutic drug monitoring. • Narrow therapeutic window that requires regular blood monitoring of plasma lithium levels and blood chemistry by a clinician to mitigate adverse events • Multiple administrations throughout the day are required to safely reach therapeutic plasma concentrations • Suffer from chronic toxicity, poor physicochemical properties and poor brain bioavailability Ionic Cocrystal (AL001) 0 2 4 6 8 10 12 14 2 hrs 24 hrs 48 hrs 72 hrs Lithium (µg/g) Plasma Lithium Levels Lithium Carbonate AL001 (LiProSal) 0 2 4 6 8 10 2 hrs 24 hrs 48 hrs 72 hrs Lithium (µg/g) Brain Lithium Levels Lithium Carbonate AL001 (LiProSal) Lithium Salicylate (salt) L - Proline (amino acid coformer) OUR SCIENCE – NON - CLINICAL
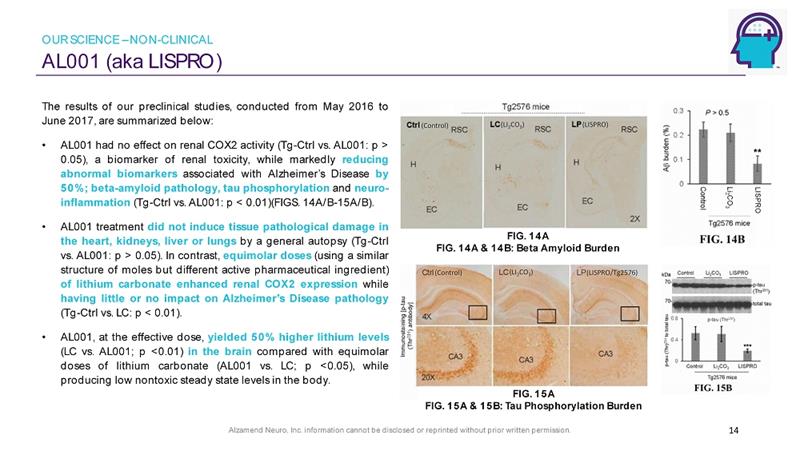
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. AL001 (aka LISPRO) 14 The results of our preclinical studies, conducted from May 2016 to June 2017 , are summarized below : • AL 001 had no effect on renal COX 2 activity ( Tg - Ctrl vs . AL 001 : p > 0 . 05 ), a biomarker of renal toxicity, while markedly reducing abnormal biomarkers associated with Alzheimer’s Disease by 50 % ; beta - amyloid pathology, tau phosphorylation and neuro - inflammation ( Tg - Ctrl vs . AL 001 : p < 0 . 01 )(FIGS . 14 A/B - 15 A/B) . • AL 001 treatment did not induce tissue pathological damage in the heart, kidneys, liver or lungs by a general autopsy ( Tg - Ctrl vs . AL 001 : p > 0 . 05 ) . In contrast, equimolar doses (using a similar structure of moles but different active pharmaceutical ingredient) of lithium carbonate enhanced renal COX 2 expression while having little or no impact on Alzheimer’s Disease pathology ( Tg - Ctrl vs . LC : p < 0 . 01 ) . • AL 001 , at the effective dose, yielded 50 % higher lithium levels (LC vs . AL 001 ; p < 0 . 01 ) in the brain compared with equimolar doses of lithium carbonate (AL 001 vs . LC ; p < 0 . 05 ), while producing low nontoxic steady state levels in the body . (LI 2 CO 3 ) (LISPRO/Tg2576) (Control) (LI 2 CO 3 ) (Control) (LISPRO) FIG. 14A FIG. 14A & 14B: Beta Amyloid Burden FIG. 15A FIG. 15A & 15B: Tau Phosphorylation Burden OUR SCIENCE – NON - CLINICAL
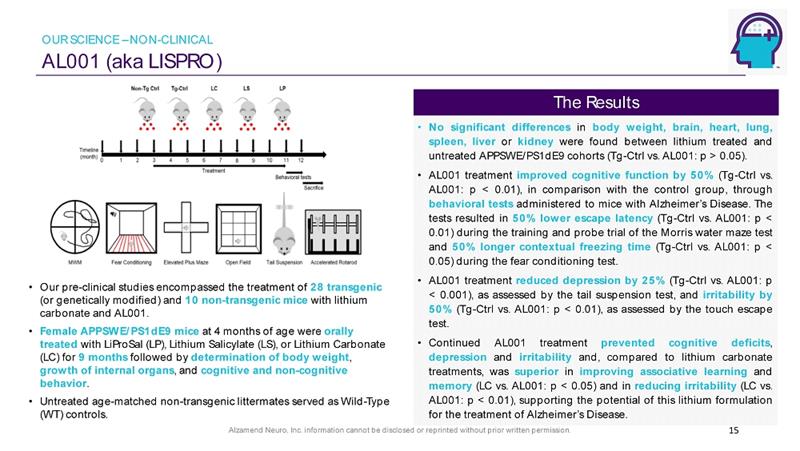
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. AL001 (aka LISPRO) 15 • Our pre - clinical studies encompassed the treatment of 28 transgenic (or genetically modified) and 10 non - transgenic mice with lithium carbonate and AL001. • Female APPSWE/PS1dE9 mice at 4 months of age were orally treated with LiProSal (LP), Lithium Salicylate (LS), or Lithium Carbonate (LC) for 9 months followed by determination of body weight , growth of internal organs , and cognitive and non - cognitive behavior . • Untreated age - matched non - transgenic littermates served as Wild - Type (WT) controls. • No significant differences in body weight, brain, heart, lung, spleen, liver or kidney were found between lithium treated and untreated APPSWE/PS 1 dE 9 cohorts ( Tg - Ctrl vs . AL 001 : p > 0 . 05 ) . • AL 001 treatment improved cognitive function by 50 % ( Tg - Ctrl vs . AL 001 : p < 0 . 01 ), in comparison with the control group, through behavioral tests administered to mice with Alzheimer’s Disease . The tests resulted in 50 % lower escape latency ( Tg - Ctrl vs . AL 001 : p < 0 . 01 ) during the training and probe trial of the Morris water maze test and 50 % longer contextual freezing time ( Tg - Ctrl vs . AL 001 : p < 0 . 05 ) during the fear conditioning test . • AL 001 treatment reduced depression by 25 % ( Tg - Ctrl vs . AL 001 : p < 0 . 001 ), as assessed by the tail suspension test, and irritability by 50 % ( Tg - Ctrl vs . AL 001 : p < 0 . 01 ), as assessed by the touch escape test . • Continued AL 001 treatment prevented cognitive deficits , depression and irritability and, compared to lithium carbonate treatments, was superior in improving associative learning and memory (LC vs . AL 001 : p < 0 . 05 ) and in reducing irritability (LC vs . AL 001 : p < 0 . 01 ), supporting the potential of this lithium formulation for the treatment of Alzheimer’s Disease . The Results OUR SCIENCE – NON - CLINICAL
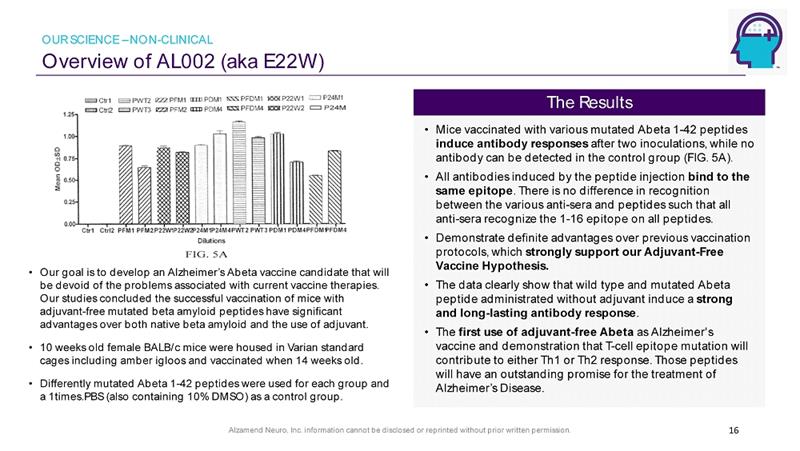
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of AL002 (aka E22W) 16 • Our goal is to develop an Alzheimer’s Abeta vaccine candidate that will be devoid of the problems associated with current vaccine therapies. Our studies concluded the successful vaccination of mice with adjuvant - free mutated beta amyloid peptides have significant advantages over both native beta amyloid and the use of adjuvant. • 10 weeks old female BALB/c mice were housed in Varian standard cages including amber igloos and vaccinated when 14 weeks old. • Differently mutated Abeta 1 - 42 peptides were used for each group and a 1times.PBS (also containing 10% DMSO) as a control group . • Mice vaccinated with various mutated Abeta 1 - 42 peptides induce antibody responses after two inoculations, while no antibody can be detected in the control group (FIG. 5A). • All antibodies induced by the peptide injection bind to the same epitope . There is no difference in recognition between the various anti - sera and peptides such that all anti - sera recognize the 1 - 16 epitope on all peptides. • Demonstrate definite advantages over previous vaccination protocols, which strongly support our Adjuvant - Free Vaccine Hypothesis. • The data clearly show that wild type and mutated Abeta peptide administrated without adjuvant induce a strong and long - lasting antibody response . • The first use of adjuvant - free Abeta as Alzheimer's vaccine and demonstration that T - cell epitope mutation will contribute to either Th1 or Th2 response. Those peptides will have an outstanding promise for the treatment of Alzheimer’s Disease. The Results OUR SCIENCE – NON - CLINICAL
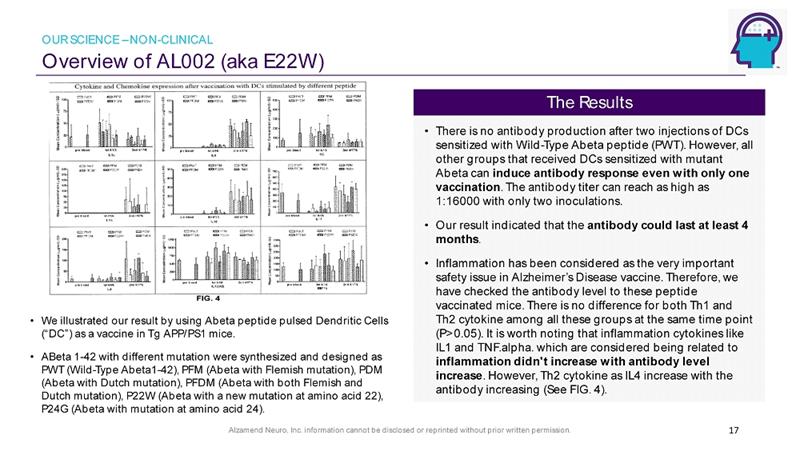
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of AL002 (aka E22W) 17 • We illustrated our result by using Abeta peptide pulsed Dendritic Cells (“DC”) as a vaccine in Tg APP/PS1 mice. • ABeta 1 - 42 with different mutation were synthesized and designed as PWT (Wild - Type Abeta1 - 42), PFM ( Abeta with Flemish mutation), PDM ( Abeta with Dutch mutation), PFDM ( Abeta with both Flemish and Dutch mutation), P22W ( Abeta with a new mutation at amino acid 22), P24G ( Abeta with mutation at amino acid 24). • There is no antibody production after two injections of DCs sensitized with Wild - Type Abeta peptide (PWT). However, all other groups that received DCs sensitized with mutant Abeta can induce antibody response even with only one vaccination . The antibody titer can reach as high as 1:16000 with only two inoculations. • Our result indicated that the antibody could last at least 4 months . • Inflammation has been considered as the very important safety issue in Alzheimer’s Disease vaccine. Therefore, we have checked the antibody level to these peptide vaccinated mice. There is no difference for both Th1 and Th2 cytokine among all these groups at the same time point (P>0.05). It is worth noting that inflammation cytokines like IL1 and TNF.alpha . which are considered being related to inflammation didn't increase with antibody level increase . However, Th2 cytokine as IL4 increase with the antibody increasing (See FIG. 4). The Results OUR SCIENCE – NON - CLINICAL
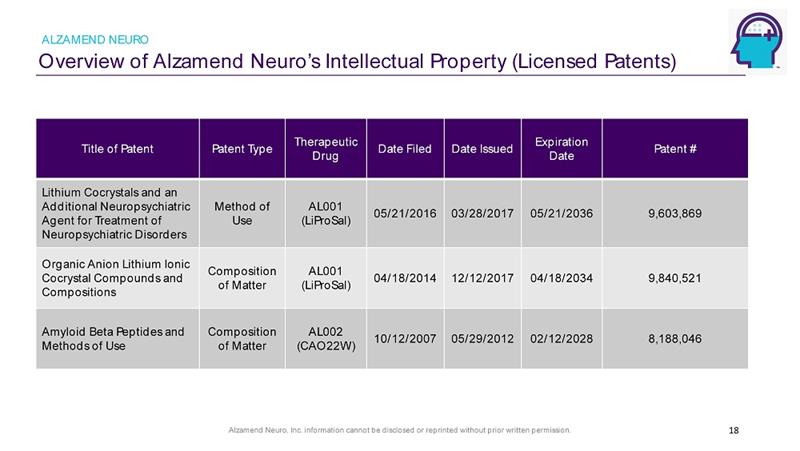
Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of Alzamend Neuro’s Intellectual Property (Licensed Patents) ALZAMEND NEURO 18 Title of Patent Patent Type Therapeutic Drug Date Filed Date Issued Expiration Date Patent # Lithium Cocrystals and an Additional Neuropsychiatric Agent for Treatment of Neuropsychiatric Disorders Method of Use AL001 (LiProSal) 05/21/2016 03/28/2017 05/21/2036 9,603,869 Organic Anion Lithium Ionic Cocrystal Compounds and Compositions Composition of Matter AL001 (LiProSal) 04/18/2014 12/12/2017 04/18/2034 9,840,521 Amyloid Beta Peptides and Methods of Use Composition of Matter AL002 (CAO22W) 10/12/2007 05/29/2012 02/12/2028 8,188,046

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of Alzheimer’s Disease Drugs on the Market COMPETITIVE LANDSCAPE 19 Aricept Exelon Namenda Razadyne *Aricept – Eisai Co., Ltd., Financial Results for Fiscal 2020(3/31/2021 for JPY FX) ( https://www.eisai.com/ir/library/settlement/pdf/e2021Q4_51.pdf ). **Exelon – 2020 Revenues for Canada & Latin America ( https://www.globenewswire.com/news - release/2021/04/23/2215925/0/en/Knight - Therapeutics - to - Acquire - Regional - Rights - to - Exelon.html ). *Namenda – Allergan 2019 Annual Report ( https://www.annualreports.com/HostedData/AnnualReports/PDF/NYSE_AGN_2019.pdf ) * Razadyne ( Reminyl ) – Takeda FY2019 Data Book(3/31/2019 for JPY FX ( https://www.takeda.com/4a24cd/siteassets/system/investors/report/quarterlyannouncements/fy2019/qr2019_q4_d_en.pdf * Aduhelm – Annual Report 2021 Fiscal Year ( https://investors.biogen.com/static - files/2c625543 - a243 - 4367 - 8248 - af360692e6d2 ) *Thomson Reuters Report - ( https://www.researchgate.net/publication/274930518_Spotlight_on_Alzheimers_disease_a_Thomson_Reuters_Pharma_Matters_report ). Year Approved: 1996 Peak Revenue Per Year: $3,454,000,000 Cost Per Patient Per Year (2022): $1,403 Total Revenue (2020): $235,000,000 Year Approved: 2000 Peak Revenue Per Year: $1,067,000,000 Cost Per Patient Per Year (2022): $5,845 Total Revenue (2020)**: $47,000,000 Year Approved: 2003 Peak Revenue Per Year: $2,575,000,000 Cost Per Patient Per Year (2022): $1,954 Total Revenue (2019): $22,800,000 Year Approved: 2004 Peak Revenue Per Year: $428,000,000 Cost Per Patient Per Year (2022): $1,715 Total Revenue (2019): $156,000,000 Aduhelm Year Approved: 2021 Peak Revenue Per Year: $3,000,000 Cost Per Patient Per Year (2022): $28,200 Total Revenue (2021): $3,000,000

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Overview of Market Opportunity for AL001 and AL002 COMPETITIVE LANDSCAPE 20 Patient Population United States Global (Including US) Major Depressive Disorder(MDD) 21 Million¹ 280 Million² PTSD 9 Million¹ 284 Million² Alzheimer ’s Disease 6.5 Million¹ 55 Million² Bipolar Disorder 7 Million¹ 45 Million² Total Patient Population 43.5 Million 664 Million Major Depressive Disorder: 1. https://www.nimh.nih.gov/health/statistics/major - depression 2. https://www.who.int/news - room/fact - sheets/detail/depression PTSD: 1. https://www.nimh.nih.gov/health/statistics/post - traumatic - stress - disorder - ptsd 2. https://www.who.int/news/item/06 - 08 - 2013 - who - releases - guidance - on - mental - health - care - after - trauma#:~:text=An%20estimated%203.6%25%20of%20the,previous%20year%2C%20the%20study%20showed Alzheimer’s: 1. https://www.alz.org/media/Documents/alzheimers - facts - and - figures.pdf 2. https://www.alzint.org/about/dementia - facts - figures/dementia - statistics/ Bipolar Disorder: 1. https://www.nimh.nih.gov/health/statistics/bipolar - disorder 2. https://www.who.int/news - room/fact - sheets/detail/mental - disorders

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend Leadership Team ALZAMEND NEURO 21 Stephan Jackman Chief Executive Officer and Director 20+ years multi - industry experience, specialized in Biotech and Pharmaceutical Lien T. Escalona Chief Financial Officer 25+ years multi - industry experience with an emphasis on accounting and finance, system implementation and SEC reporting Kenneth S. Cragun Senior Vice President of Finance 30+ years SEC reporting, CFO of publicly - traded company on Nasdaq, multi - industry experience, including Biotech and Healthcare Henry Nisser Executive Vice President, General Counsel and Director 20+ years experience, U.S. securities compliance, M&A, equity/debt financings and corporate governance David J. Katzoff Chief Operating Officer 30+ years multi - industry experience, including Healthcare and Technology

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Alzamend Scientific Advisory Board ALZAMEND NEURO 22 Thomas M. Wisniewski, MD Director, NYU Langone’s Pearl I. Barlow Center for Memory Evaluation and Treatment 300+ Peer - Reviewed Medical Journal Publications (19 U.S. Patents Issued) Leads a Research Laboratory Continuously Funded by the National Institutes of Health for 20+ Years Eric McDade , DO Associate Director, DIAN Trials Unit & Clinical Trials Leadership, Washington University School of Medicine Associate Professor of Neurology, Washington University School of Medicine 157+ Peer - Reviewed Journal Publications Terri Hunter, Ph.D. Technology Transfer and Partnerships Specialist, U.S. Department of Veterans Affairs 20+ years Experience, Research and Technology Transfer and Partnerships Ph.D. in Medical Sciences from the University of South Florida College of Medicine

Alzamend Neuro, Inc. information cannot be disclosed or reprinted without prior written permission. Lynne Fahey McGrath, Ph.D. Regulatory Affairs and Product Development Consultant 30+ years experience, Biotech and Pharmaceuticals M.P.H./Ph.D., Public Health from UMDNJ – Robert Wood Johnson Medical School Jeffrey Oram Principal at Godby Realtors 25+ years multi - industry experience, Investments, Real Estate and Technology Andrew H. Woo M.D., Ph.D. Practicing physician at Santa Monica Neurological Consultants, Assistant Clinical Professor of Neurology at the David Geffen School of Medicine at UCLA and Cedars - Sinai Medical Center 20+ years experience in Psychiatry and Neurology Mark Gustafson C.P.A. Chief Financial Officer of PharmaKure Limited 30 + years multi - industry experience as an active CPA, specialized in Biotech, Energy and Technology Alzamend Board of Directors 23 William B. Horne Chairman of Alzamend Chief Executive Officer of BitNile Holdings 25+ years Financial Industry experience, prior “Big 4” auditor and healthcare executive Stephan Jackman Chief Executive Officer and Director 20+ years multi - industry experience, specialized in Biotech and Pharmaceutical Henry Nisser Executive Vice President, General Counsel and Director 20+ years experience, U.S. securities compliance, M&A, equity/debt financings and corporate governance FINAL DETAILS

CONTACT US 3500 Lenox Road, Suite 1500 Atlanta, GA 30326 +1 (844) 722 - 6333 Info@alzamend.com www.alzamend.com April 2022