UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended
For the transition period from _______________ to _______________
Commission file number
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
| ( | ||
| (Address of principal executive offices) | (Zip Code) | (Registrant’s telephone number, including area code) |
Securities registered under Section 12(b) of the Act:
| Title of Each Class | Trading Symbol | Name of each exchange on which registered |
Securities registered under Section 12(g) of the Act: None
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange
Act. Yes ¨
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities
Exchange Act of 1934 during the preceding year (or for such shorter period that the registrant was required to file such reports), and
(2) has been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ |
| Smaller reporting company | |
| Emerging growth company |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ¨
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨
The aggregate market value of the common stock
held by non-affiliates of the registrant, based on the closing price of the shares of common stock on October 31, 2021 (the last business
day of the registrant’s most recently completed second fiscal quarter), as reported by The Nasdaq Stock Market LLC on such date
was approximately $
There were shares of common stock outstanding as of July 19, 2022.
Documents incorporated by reference: None
ALZAMEND NEURO, INC.
FORM 10-K
FOR THE FISCAL YEAR ENDED APRIL 30, 2022
INDEX
| Page | |||
| PART I | |||
| Item 1. | Business | 2 | |
| Item 1A. | Risk Factors | 22 | |
| Item 1B. | Unresolved Staff Comments | 47 | |
| Item 2. | Properties | 47 | |
| Item 3. | Legal Proceedings | 47 | |
| Item 4. | Mine Safety Disclosures | 47 | |
| PART II | |||
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
48 | |
| Item 6. | [Reserved] | 48 | |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 49 | |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 59 | |
| Item 8. | Financial Statements and Supplementary Data | 59 | |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 59 | |
| Item 9A. | Controls and Procedures | 59 | |
| Item 9B. | Other Information | 61 | |
| Item 9C. | Disclosures Regarding Foreign Jurisdictions that Prevent Inspections | 61 | |
| PART III | |||
| Item 10. | Directors, Executive Officers and Corporate Governance | 62 | |
| Item 11. | Executive Compensation | 67 | |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management | 73 | |
| Item 13. | Certain Relationships and Related Transactions and Director Independence | 75 | |
| Item 14. | Principal Accountant Fees and Services | 77 | |
| PART IV | |||
| Item 15. | Exhibit and Financial Statement Schedules | 78 | |
| Item 16. | Form 10-K Summary | 79 | |
| Signatures | 80 |
NOTE ABOUT FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K (the “Annual Report”) contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements relate to future events or our future financial performance. We have attempted to identify forward-looking statements by terminology including “anticipates,” “believes,” “expects,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predict,” “should” or “will” or the negative of these terms or other comparable terminology. These statements are only predictions; uncertainties and other factors may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels or activity, performance or achievements expressed or implied by these forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Our expectations are as of the date this Annual Report is filed, and we do not intend to update any of the forward-looking statements after the date this Annual Report is filed to confirm these statements to actual results, unless required by law.
This Annual Report also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other industry data. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified the statistical and other industry data generated by independent parties and contained in this Annual Report and, accordingly, we cannot guarantee their accuracy or completeness, though we do generally believe the data to be reliable. In addition, projections, assumptions and estimates of our future performance and the future performance of the industries in which we operate are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors” and elsewhere in this Annual Report. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
RISK FACTOR SUMMARY
Below is a summary of the principal factors that make an investment in our common stock speculative. This summary does not address all of the risks that we face. Additional discussion of the risks summarized in this risk factor summary, and other risks that we face, can be found below under the heading “Risk Factors” and should be carefully considered, together with other information in this Annual Report and our other filings with the Securities and Exchange Commission (the “SEC”), before making investment decisions regarding our common stock.
| · | We are at an early clinical-stage of development and currently have no source of near-term revenue and may never become profitable. |
| · | We have a limited operating history on which to judge our business prospects and management. |
| · | We will need, but may be unable to obtain, funding on satisfactory terms, which could dilute our stockholders and investors, and/or impose burdensome financial restrictions on our business. |
| · | We have both operational and financial milestones that must be met to maintain the licensing rights to our current technology and intellectual property from the University of South Florida Research Foundation. |
| · | If we fail to comply with our obligations in the agreements under which we license intellectual property and other rights from third parties or otherwise experience disruptions to our business relationships with the Licensor, we could lose license rights that are important to our business. |
| · | We are substantially dependent on the success of our product candidates, which may not receive regulatory approval or be successfully commercialized. |
| · | Serious adverse events or other safety risks could require us to abandon development and preclude, delay or limit approval of AL001 or AL002, or limit the scope of any approved label or market acceptance. |
| · | Development and regulatory approval of our drug candidates present a number of risks, which are delineated in the Risk factors section. |
| · | If we fail to attract and keep senior management and key scientific personnel, we may be unable to successfully develop AL001, AL002 or any future product candidates, conduct our in-licensing and development efforts or commercialize AL001, AL002 or any of our future product candidates. |
| · | Our intellectual property rights present a number of risks, which are delineated in the Risk factors section. |
| · | Our affiliates and related party transactions present a number of risks, which are delineated in the Risk factors section. |
| · | If we do not continue to satisfy the Nasdaq Capital Market continued listing requirements, our common stock could be delisted from the Nasdaq Capital Market. |
| · | The market price of our common stock is volatile, which could result in substantial losses for investors. |
| · | The concentration of our stock ownership will limit your ability to influence corporate matters, including the ability to influence the outcome of director elections and other matters requiring stockholder approval. |
| · | We have identified a material weakness in our internal control over financial reporting. If our remediation of this material weakness is not effective, or if we experience additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls in the future, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, the value of our common stock. |
PART I
| ITEM 1. | BUSINESS |
In this Annual Report, unless the context requires otherwise, references to the “Company,” “Alzamend,” “we,” “our company” and “us” refer to Alzamend Neuro, Inc., a Delaware corporation.
Company Overview
We are an early clinical-stage biopharmaceutical company focused on developing novel products for the treatment of Alzheimer’s disease (“Alzheimer’s”), bipolar disorder, major depressive disorder (“MDD”) and post-traumatic stress disorder (“PTSD”). With our two product candidates, we aim to bring treatments or cures to market as quickly as possible. Far too many individuals, patients and caregivers suffer from the burden created by these devastating, and often fatal, diseases. Our primary target, Alzheimer’s, was among the most-feared diseases (second only to cancer) among Americans, according to a 2011 survey by the Harvard School of Public Health. Alzheimer’s is also the sixth leading cause of death in the United States according to a 2021 report from the Alzheimer’s Association, a nonprofit that funds research. Existing Alzheimer’s treatments only temporarily relieve symptoms but do not slow or halt the progression of the disease, which currently affects roughly 6.2 million Americans and that number is expected to grow to 13 million individuals by 2050. Alzheimer’s also impacts more than 11 million Americans who provide an estimated 16 billion hours of unpaid care per year, valued at $272 billion, according to data provided by the Alzheimer’s Association. In 2022, the estimated healthcare costs for treating individuals with Alzheimer’s in the United States will be $321 billion, including $206 billion in Medicare and Medicaid payments. These costs could rise to as high as $1 trillion per year by 2050 if no permanent treatment or cure for Alzheimer’s is found, the Alzheimer’s Association reported.
Our pipeline consists of two novel therapeutic drug candidates:
| · | AL001 - A patented ionic cocrystal technology delivering a therapeutic combination of lithium, proline and salicylate, known as AL001, through two royalty-bearing exclusive worldwide licenses from the University of South Florida Research Foundation, Inc., as licensor (the “Licensor”); and |
| · | AL002 - A patented method using a mutant peptide sensitized cell as a cell-based therapeutic vaccine that seeks to restore the ability of a patient’s immunological system to combat Alzheimer’s, known as AL002 or CA022W, through a royalty-bearing exclusive worldwide license from the Licensor. |
Our most advanced product candidate (lead product) licensed and in clinical development in humans is an ionic cocrystal of lithium for the treatment of Alzheimer’s, bipolar disorder, MDD and PTSD. Based on our preclinical data, AL001 treatment prevents cognitive deficits, depression and irritability in APPSWE/PS1dE9 mice, and is superior in improving associative learning and memory and irritability compared with lithium carbonate treatments, supporting the potential of this lithium formulation for the treatment of Alzheimer’s, bipolar disorder, MDD and PTSD in humans. Lithium has been marketed for more than 35 years and human toxicology regarding lithium use has been well characterized, potentially mitigating the regulatory burden for safety data.
The results of randomized, placebo-controlled, clinical trials of lithium in the treatment of patients with Alzheimer’s dementia and subjects with mild cognitive impairment have been widely published. Clinical studies have indicated that lithium administered at doses lower than those used for affective disorders can favorably impact Alzheimer’s outcomes. A study by O.V. Forlenza, et al., entitled “Disease-Modifying Properties of Long-Term Lithium Treatment for Amnestic Mild Cognitive Impairment: Randomized Controlled Trial,” appearing in the British Journal of Psychiatry (2011) reported that lithium was superior to a placebo, evidencing a slower decline of cognitive function as measured by the Alzheimer’s Disease Assessment Scale cognitive subscale. Given the absence of adequate treatments that can slow, halt or even reverse the decline of this highly prevalent disease, the potential efficacy of lithium in the long-term management of Alzheimer’s may positively impact public health. There is an unmet medical need for safe and effective Alzheimer’s treatments, particularly for treatments with neuroprotective properties.
There is increasing evidence to suggest that depressive illness, particularly in the elderly, is associated with neuronal cell loss. These findings suggest that lithium may exert some of its long-term beneficial effects in the treatment of affective disorders via underappreciated neuroprotective effects. Molecular biology and animal studies have also suggested that lithium may offer protection against Alzheimer’s. Given the absence of other adequate treatments, the potential efficacy of lithium in the long-term treatment of neurodegenerative disorders may be warranted.
| - 2 - |
Following Phase III clinical trials in humans, we intend to seek approval to commercialize AL001 via a New Drug Application (“NDA”). As one of the initial steps of the NDA process, we submitted a pre-Investigational New Drug (“pre-IND”) briefing package to the U.S. Food and Drug Administration (“FDA”) in July 2019 that argued against the need for any further preclinical safety studies. We submitted an Investigational New Drug (“IND”) application to the FDA on June 30, 2021. On July 28, 2021, the FDA responded to our IND and stated that we may proceed with Phase I clinical trials in humans. We initiated our Phase I clinical trial on September 13, 2021. We completed our Phase I clinical trial in March 2022 and initiated a Phase IIA clinical trial in May 2022.
The ongoing Phase IIA study is evaluating the safety and tolerability of AL001 under multiple-dose, steady-state conditions and is determining the maximum tolerated dose in patients diagnosed with mild to moderate Alzheimer’s. The lithium and salicylate components of AL001 are to be given within the amounts already approved for use in patients for other indications. Up to 40 subjects will complete the Phase IIA trial. The maximum tolerated dose will then be used for further studies. Topline data are expected in December 2022 from this study.
Based on our preclinical data, AL001 has a positive effect on the pharmacodynamic biomarkers of Alzheimer’s. We propose to validate this clinically and if confirmed, we believe that AL001 is a candidate for breakthrough therapy designation because of its positive effect on a pharmacodynamic biomarker (beta-amyloids) and potential for a clinically meaningful effect on Alzheimer’s. A drug that receives a breakthrough therapy designation is eligible for fast-track designation features, intensive guidance on an efficient drug development program and FDA organizational commitment involving senior managers. However, we have not yet applied for breakthrough therapy designation nor have we received any official designation for expedited development. Our product candidate may not qualify for breakthrough therapy designation; further, even if it does qualify for breakthrough therapy designation, it may not actually lead to faster development or expedited regulatory review and approval or necessarily increase the likelihood that it will receive FDA approval.
Additionally, we believe that AL001 is positioned for an expedited Section 505(b)(2) regulatory pathway for new drug. AL001’s active pharmaceutical ingredients (lithium, proline and salicylate) are well documented and approved by the FDA. The provisions of 505(b)(2) were created, in part, to help avoid unnecessary duplication of studies already performed on a previously approved (“reference” or “listed”) drug. This section gives the FDA express permission to rely on data not developed by the NDA applicant. This process can result in a much less expensive and much faster route to approval, compared with a traditional development path such as 505(b)(1), while creating new, differentiated products with tremendous commercial value. If we successfully obtain a breakthrough therapy designation and the Section 505(b)(2) regulatory pathway for new drug approvals, we believe we can shorten the development timeline for AL001. However, our product candidate may not qualify for expedited development or, if it does qualify for expedited development, it may not actually lead to faster development or expedited regulatory review and approval.
We believe that our ability to re-engineer lithium solid dosage forms in order to optimize performance has the potential to address a wide range of clinical applications ranging from neurodegenerative disorders, not merely Alzheimer’s, but also amyotrophic lateral sclerosis (known as ALS and Lou Gehrig’s disease), Huntington’s disease, multiple sclerosis, Parkinson’s disease and traumatic brain injury, to more psychiatric conditions such as bipolar disorder, MDD, mania, PTSD and suicidality. This novel approach is intended to achieve the desired therapeutic outcome of enhanced penetration through the blood-brain barrier and sustained brain lithium concentrations while systemic exposures (and toxicities) are mitigated for other organ systems. The optimal modified-release lithium dosing approach should avoid acutely toxic peak concentrations in blood, as well as in the brain, and should maintain such blood concentrations for a predictable, clinically relevant time, with overall low systemic exposures that mitigate the potential for adverse events. We anticipate that the lithium delivery system will be adaptable to a dosing regimen that maintains therapeutic brain lithium concentrations consistently for the longest possible time while allowing only modest exposures and providing adequate recovery periods between doses for other organ systems.
We have an additional preclinical candidate for Alzheimer’s, AL002, which has transitioned from early-stage development to an extensive program of preclinical study and evaluation that was completed on May 31, 2021, and was followed by a comprehensive report prepared by Charles River Laboratories, Inc., an independent preclinical service provider, received on July 23, 2021. We submitted a pre-IND meeting request for AL002 and supporting briefing documents to the Center for Biological Evaluation and Research of the FDA on July 30, 2021. We received a written response to relating to the pre-IND from the FDA providing a path for Alzamend’s planned clinical development of AL002 on September 30, 2021. The FDA agreed to allow Alzamend to submit an IND to conduct a combined Phase I/II study. We anticipate submitting an IND to initiate a Phase I/II study for AL002 in September 2022.
Our Business Strategy
We intend to develop and commercialize therapeutics that are better than existing treatments and have the potential to significantly improve the lives of individuals afflicted by Alzheimer’s, bipolar disorder, MDD and PTSD. To achieve these goals, we are pursuing the following key business strategies:
| • | Advance clinical development of AL001 and AL002 for Alzheimer’s treatment. For our lead candidate, AL001, we completed our Phase I clinical trial in March 2022 and initiated a Phase IIA clinical trial in May 2022. We anticipate topline data in December 2022 from our ongoing Phase IIA multiple ascending dose (“MAD”) clinical trial for AL001 treatment of dementia related to Alzheimer’s. We completed our preclinical study and evaluation of AL002 on May 31, 2021, and anticipate submitting an IND to initiate a Phase I/II study in September 2022; |
| - 3 - |
| • | Expand our pipeline of pharmaceuticals to include additional indications for AL001 and delivery methods. Another element of our business strategy is to expand our pipeline of pharmaceuticals based on our technology and advance these product candidates through clinical development for the treatment of a variety of indications. In addition to treating Alzheimer’s, AL001 has the potential to treat a wide range of neurodegenerative diseases and psychiatric disorders. We plan to pursue the treatment of bipolar disorder, MDD, and PTSD with AL001, and in May 2022, we submitted a pre-IND meeting request to the FDA for these indications and received a written response from the FDA in July 2022. Based on the written response from the FDA, we plan to submit separate INDs for bipolar disorder, MDD, and PTSD after completion of the current Phase II MAD clinical trial, which would allow us to initiate Phase II studies in each of those indications. We also plan to explore different formulations (liquid, immediate release and sprinkle capsules) to deliver AL001; |
| • | Focus on translational and functional endpoints to efficiently develop product candidates. We believe AL001 is positioned for a Section 505(b)(2) regulatory pathway for new drug approvals. We also believe AL001 and AL002 are positioned for breakthrough therapy designations because of their positive effects on a pharmacodynamic biomarker (beta-amyloids) and potential for a clinically meaningful effect on Alzheimer’s, making them eligible to receive assistance from the FDA throughout the development process that may shorten the development timelines. However, we have neither received breakthrough therapy designation nor have we qualified for expedited development, and no assurance can be given that we will. Even if we qualify for breakthrough therapy designation or expedited development, it may not actually lead to faster development or expedited regulatory review and approval or necessarily increase the likelihood that we will receive FDA approval; and |
| • | Optimize the value of AL001 and AL002 in major markets. We intend to commercialize AL001 and AL002 by seeking FDA marketing approval for both product candidates and partnering with biopharmaceutical companies seeking to strategically fortify pipelines and, in turn, receiving funding for the costly later-stage clinical development. We do not anticipate selling products directly into the marketplace, though we may do so depending on market conditions. Our focus is expected to concentrate on entering into strategical transactions with established distributors and producers, which will provide distribution and marketing capabilities for the sale of our products into the marketplace. |
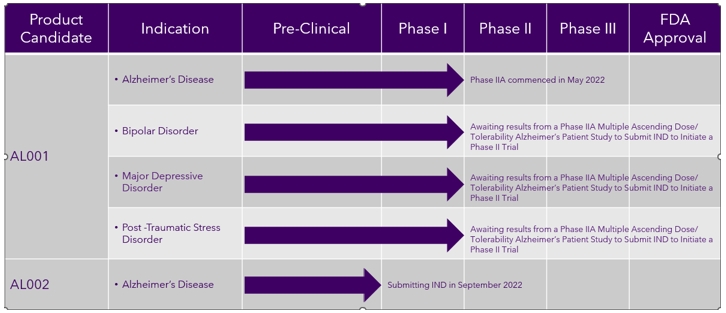
Our Development Pipeline
The following chart provides an overview of the current development stages of our therapeutic product candidates.

Our Proprietary Technology AL001 Drug Candidate
Our lead product candidate that we have licensed and have begun clinical development in humans is an ionic cocrystal of lithium for the treatment of Alzheimer’s, bipolar disorder, MDD and PTSD. Lithium salts have a long history of human consumption beginning in the 1800s. In psychiatry, they have been used to treat mania and as a prophylactic for depression since the mid-20th century. Today, lithium salts are used as a mood stabilizer for the treatment of bipolar disorder. Although the FDA has approved no medications as safe and effective treatments for suicidality, lithium has proven to be the only drug that consistently reduces suicidality in patients with neuropsychiatric disorders. Despite these effective medicinal uses, current FDA-approved lithium pharmaceutics (lithium carbonate and lithium citrate) are limited by a narrow therapeutic window that requires regular blood monitoring of plasma lithium levels and blood chemistry by a clinician to mitigate adverse events. Because conventional lithium salts (carbonate and citrate) are eliminated relatively quickly, multiple administrations throughout the day are required to safely reach therapeutic plasma concentrations. Existing lithium drugs, such as lithium chloride and lithium carbonate, suffer from chronic toxicity, poor physicochemical properties and poor brain bioavailability. Because lithium is so effective at reducing manic episodes in patients with bipolar disorder, it is still used clinically despite its narrow therapeutic index. This has led researchers to begin to look for alternatives to lithium with similar bioactivities.
| - 4 - |
Scientists from the University of South Florida have developed a new lithium cocrystal composition and method of preparation that, under certain clinical and/or testing conditions, have been shown to allow for lower dosages to achieve therapeutic brain levels of lithium for psychiatric disorders, which could lead to a broadening of lithium’s therapeutic index. Our studies and/or testing have indicated that the compound offers improved physiochemical properties compared to existing forms of lithium, giving it the potential to be developed as an anti-suicidal drug and for use against mood disorders.
Recent evidence suggests that lithium may be efficacious for both the treatment and prevention of Alzheimer’s. Unlike traditional medications which only address a single therapeutic target, lithium appears to be neuroprotective through several modes of action. For example, recent studies have indicated that it exerts neuroprotective effects, in part, by increasing a brain-derived neurotrophic factor leading to restoration of learning and memory. Another neuroprotective mechanism of lithium indicated by recent studies is the attenuation of the production of inflammatory cytokines like IL-6 and nitric oxide in activated microglia. Results from recent clinical studies suggest that lithium treatment may reduce dementia development while preserving cognitive function and reducing biomarkers associated with Alzheimer’s.
The novel ionic cocrystal of lithium (AL001), which was designed, synthesized and characterized by a team of inventors from the University of South Florida has been shown to exhibit improved nonclinical pharmacokinetics compared to currently FDA-approved lithium products and is also bioactive in many in vitro models of Alzheimer’s. AL001 may constitute a means of treating Alzheimer’s, bipolar disorder, MDD and PTSD. Our preclinical studies encompassed the treatment of 28 transgenic (or genetically modified) and 10 non-transgenic mice with lithium carbonate and AL001. In particular, female APPSWE/PS1dE9 mice at 4 months of age were fed with either regular chow (Tg-Ctrl, n=8) or chow that contained lithium carbonate (LC, 0.05% equivalent to 83 mg/kg/day, n=6), or lithium salicylate (LS, 0.20% equivalent to 325 mg/kg/day, n=6), or lithium salicylate proline co-crystal, AL001 (AL001, 0.35% equivalent to 583 mg/kg/day, n=8) for 9 months. In addition, aged-matched non- transgenic background control mice (B6C3F1/J, Non-Tg-Ctrl, n=10) were fed regular chow for 9 months as control. Each treatment group was subject to a battery of behavioral tests at 12 months of age and mice were sacrificed at 13 months of age. The results of our preclinical studies, conducted from May 2016 to June 2017, are summarized below:
| • | AL001 treatment improved cognitive function by 50% (Tg-Ctrl vs. AL001: p<0.01), in comparison with the control group, through behavioral tests administered to mice with Alzheimer’s. The tests resulted in 50% lower escape latency (Tg-Ctrl vs. AL001: p<0.01) during the training and probe trial of the Morris water maze test and 50% longer contextual freezing time (Tg-Ctrl vs. AL001: p<0.05) during the fear conditioning test; |
| • | AL001 treatment reduced depression by 25% (Tg-Ctrl vs. AL001: p<0.001), as assessed by the tail suspension test, and irritability by 50% (Tg-Ctrl vs. AL001: p<0.01), as assessed by the touch escape test; |
| • | In comparison with lithium carbonate treatment, AL001 treatment afforded superior protection against cognitive impairment by 50% (LC vs. AL001; p<0.05), as shown by the contextual fear conditioning test, and irritability by 50% (LC vs. AL001: p<0.01); |
| • | Continued AL001 treatment prevented cognitive deficits, depression and irritability and, compared to lithium carbonate treatments, was superior in improving associative learning and memory (LC vs. AL001: p<0.05) and in reducing irritability (LC vs. AL001: p<0.01), supporting the potential of this lithium formulation for the treatment of Alzheimer’s; |
| • | AL001 had no effect on renal COX2 activity (Tg-Ctrl vs. AL001: p>0.05), a biomarker of renal toxicity, while markedly reducing abnormal biomarkers associated with Alzheimer’s by 50%, in particular beta-amyloid pathology, tau phosphorylation and neuro-inflammation (Tg-Ctrl vs. AL001: p<0.01); |
| • | AL001 treatment did not induce tissue pathological damage in the heart, kidneys, liver and lungs by a general autopsy (Tg-Ctrl vs. AL001: p>0.05). In contrast, equimolar doses (using a similar structure of moles but different active pharmaceutical ingredient) of lithium carbonate enhanced renal COX2 expression while having little or no impact on Alzheimer’s pathology (Tg-Ctrl vs. LC: p<0.01); |
| • | AL001, at the effective dose, yielded 50% higher lithium levels (LC vs. AL001; p<0.01) in the brain compared with equimolar doses of lithium carbonate (AL001 vs. LC; p<0.05), while producing low nontoxic steady state levels in the body; and |
| • | No significant differences in body weight, brain, heart, lungs, spleen, liver or kidneys were found between cohorts treated with AL001 and untreated cohorts. (Tg-Ctrl vs. AL001: p>0.05). |
| - 5 - |
In analyzing the preclinical study results, a p-value is used to determine the probability as to whether the difference between two data sets is due to chance. The smaller the p-value, the more likely the differences are not due to chance alone. In general, if the p-value is less than or equal to 0.05, the outcome is considered statistically significant. The FDA’s evidentiary standard of efficacy generally relies on a p-value of less than or equal to 0.05. A p-value greater than 0.05 is considered statistically non-significant. As shown above, all of the results of our preclinical studies were statistically significant compared to the control group.
On September 13, 2021, we initiated a randomized, balanced, Phase I, single-dose, open-label, two-treatment, two-period, two- sequence, crossover, relative bioavailability study to investigate lithium pharmacokinetics and safety of AL001 formulation compared to a marketed immediate release lithium carbonate formulation in healthy subjects. The primary objective of this study was to assess the relative bioavailability of the AL001 lithium formulation relative to a marketed lithium carbonate formulation in healthy subjects for the purpose of determining potential clinically safe and effective AL001 dosing in future studies. Additionally, we wanted to characterize safety and tolerability of the tested formulations under the conditions of this study. This was a first-in-human study of the AL001 formulation; this trial was designed to assess the relative bioavailability of the AL001 lithium formulation compared to a marketed lithium carbonate formulation in at least 24 completed healthy subjects (30 subjects were to be enrolled) for the purpose of determining potential clinically safe and effective AL001 dosing in future studies. The AL001 lithium content was nearly half of the reference lithium carbonate capsule dosage as it was expected that treatment of frail Alzheimer’s patients will require half the lithium dose used for treatment of bipolar/affective disorders. Lithium carbonate 300 mg (Reference product) was given as a single dose in this study; this is often used as a starting dose for treatment of bipolar/affective disorders when given three times daily. The shape of the AL001 lithium plasma concentration versus time curve was unknown prior to this study. Also unknown were the AL001 rate and extent of lithium absorption. The Phase I study was completed in March 2022 with the following results:
| · | AL001 was shown to be safe and well-tolerated in healthy adult subjects; |
| · | No serious adverse events and no deaths were reported during the trial; |
| · | The safety profiles of both AL001 and the marketed lithium carbonate capsule were benign; |
| · | No clinically significant abnormal findings in electrocardiograms were noted during the trial; |
| · | AL001 salicylate plasma concentrations were observed to be well tolerated and consistently within safe limits; and |
| · | Dose-adjusted relative bioavailability analyses of the rate and extent of lithium absorption in plasma indicated that AL001 1050 mg (lithium content equivalent to 150 mg lithium carbonate) is bioequivalent to a marketed 300 mg lithium carbonate capsule and the shapes of the lithium plasma concentration versus time curves are similar. |
On May 5, 2022, we initiated a multiple-dose, steady-state, double-blind, ascending dose safety, tolerability, pharmacokinetic study (www.clinicaltrials.gov, identifier: NCT05363293) of AL001 in patients with mild to moderate Alzheimer’s with the following objectives:
| · | Primary: To evaluate the safety and tolerability of AL001 under multiple-dose, steady-state conditions in Alzheimer’s patients; |
| · | Secondary: To characterize the maximum tolerated dose (MTD) of AL001 in patients with mild to moderate Alzheimer’s; and |
| · | Exploratory: Determination of qualitative and quantitative evaluations of AD patient desirable characteristics for future Phase 2 and 3 clinical studies in order to: |
| o | Facilitate recruitment into subsequent AL001 clinical trials; and |
| o | Facilitate trial-adherence to completion of study requirements including treatment adherence. |
A product can be designated as a breakthrough therapy if it is intended to treat a serious condition (for which the FDA considers Alzheimer’s) and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s). For purposes of breakthrough therapy designation, a clinically significant endpoint generally refers to an endpoint that measures an effect on irreversible morbidity or mortality (“IMM”), or on symptoms that represent serious consequences of the disease. A clinically significant endpoint can also refer to findings that suggest an effect on IMM or serious symptoms, including:
| • | an effect on an established surrogate endpoint; |
| • | an effect on a surrogate endpoint or intermediate clinical endpoint considered reasonably likely to predict a clinical benefit (i.e., the accelerated approval standard); |
| - 6 - |
| • | an effect on a pharmacodynamic biomarker (which is a measurable indicator of the disease state) that does not meet criteria for an acceptable surrogate endpoint, but strongly suggests the potential for a clinically meaningful effect on the underlying disease; and |
| • | a significantly improved safety profile compared to available therapy (e.g., less dose-limiting toxicity for an oncology agent), with evidence of similar efficacy. |
Based on our preclinical data, AL001 has a positive effect on the pharmacodynamic biomarkers of Alzheimer’s. As a result, we believe that if the data from AL001 in the aforementioned Phase II study provides confirmation of the pre-clinical data, i.e., positive effect on a pharmacodynamic biomarker (beta-amyloids) and potential for a clinically meaningful effect on Alzheimer’s, AL001 will be a candidate for breakthrough therapy designation. If breakthrough therapy designation is granted, it would be eligible for intensive guidance on an efficient drug development program and FDA organizational commitment involving senior managers. However, we have neither received breakthrough therapy designation nor have we qualified for expedited development. Our product candidate may not qualify for breakthrough therapy designation; further, even if it does qualify for breakthrough therapy designation, it may not actually lead to faster development or expedited regulatory review and approval or necessarily increase the likelihood that it will receive FDA approval.
Additionally, we believe that AL001 is positioned for an expedited Section 505(b)(2) regulatory pathway for new drug. AL001’s active pharmaceutical ingredients (lithium, proline and salicylate) are well documented and approved by the FDA. The provisions of Section 505(b)(2) were created, in part, to help avoid unnecessary duplication of studies already performed on a previously approved (“reference” or “listed”) drug. This section gives the FDA express permission to rely on data not developed by the NDA applicant. This can result in a much less expensive and much faster route to approval, compared with a traditional development path such as Section 505(b)(1), while creating new, differentiated products with tremendous commercial value. If we successfully obtain a breakthrough therapy designation and the Section 505(b)(2) regulatory pathway for new drug approvals, we believe we can shorten the development timeline for AL001. However, our product candidate may not qualify for expedited development or, if it does qualify for expedited development, it may not actually lead to faster development or expedited regulatory review and approval.
AL001 will require extensive clinical evaluation, regulatory review and approval, significant marketing efforts and substantial investment before it or any successors are likely to provide us with any revenue. As a result, if we do not successfully develop, achieve regulatory approval for and commercialize AL001, our long-term business plans will not be met, and we will be unable to generate the revenue we have forecast for the foreseeable future, if any. We do not anticipate that we will generate our maximum revenue for several years, or that we will achieve profitability for this therapeutic drug candidate until at least a few years after generating material revenue, if at all. If we are unable to generate revenue or raise substantial additional capital, we will not be able to pursue any expansion of our business or acquire additional intellectual property, we will not become profitable with this therapeutic drug candidate, and we will be unable to continue our operations at the currently planned pace, if at all.
AL002 Drug Candidate
The other product candidate that we have licensed to clinically develop in humans is AL002, a patented method using a mutant peptide sensitized cell as a cell-based therapeutic vaccine which seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s. The proposed mechanism of action is through the pulsed-Dendritic Cell (“DC”) activation of T-cells that stimulates the immune system, resulting in the clearance of brain amyloid. Preclinical studies conducted from April 2005 to July 2010 suggest that the infusion of transgenic (or genetically modified) mice with AL002-pulsed DCs is associated with lower amyloid burden and improved neuro-behavioral performance. This is likely to be mediated by an anti-inflammatory effect in addition to the immunogenicity of this therapy.
AL002 is based on the theory that Alzheimer’s symptoms may be caused in large part by plaque deposits that can cluster in the brain composed of protein fragments called beta-amyloids that build up between nerve cells. One hypothesis is that a special type of immune cell, natural beta-amyloid antibodies, may play a role in preventing plaque build-up in people without Alzheimer’s. As people age, their immune systems may degrade, and some people may be unable to produce natural beta-amyloid antibodies, the absence of which leads to the plaque build-up causing Alzheimer’s.
AL002 is intended to elicit an immune response to produce anti-amyloid antibodies, which can then neutralize circulated beta-amyloids and prevent additional plaque build-up. The mutant antigen within AL002 was selected specifically for its high HLA binding affinity, thereby avoiding the need for an adjuvant, which may cause an adverse (Th1) immune response.
AL002 is an autologous modified DC treatment. More precisely, it is a patient-specific therapy where the patient undergoes leukapheresis, a nonsurgical treatment used to reduce the quantity of white blood cells in the bloodstream, to isolate peripheral blood monocytes that are subsequently matured into DCs using an IL4+ GM-CSF cocktail. The DCs are incubated with a modified amyloid beta (Aβ) peptide (“AL002 peptide”) to sensitize them, and then administered to the same patient.
| - 7 - |
Significant evidence has accumulated recently suggesting that immunotherapy is a highly promising modality of treatment in Alzheimer’s. Most current immune-based active investigations are focused on passive immunization by pre-prepared Aβ antibody administration. Active immunization may offer additional or more lasting effects on the clearance of amyloid and a safer approach due to its reliance on autologous immune mechanisms. Further, preliminary evidence suggests a recurrence of the amyloid accumulation after clearance with the immunoglobulins. A prior attempt at engaging the immune system to treat Alzheimer’s was conducted using the immunization with pre-aggregated synthetic Aβ (AN-1792) combined with the immunogenic adjuvant QS-21. The Phase IIA study with AN-1792 was terminated by the FDA due to severe meningoencephalitis in approximately 6% of vaccinated subjects. We believe that this may have been caused by using a strong non-specific antigenic determinant T-cell epitope in the Aβ 1-42 peptide and the inclusion of a QS21 adjuvant and polysorbate-80 stabilizing agent in the vaccine formulation.
On July 23, 2021, we announced that Alzamend received positive toxicology results for AL002 in a good laboratory practices (“GLP”) toxicology study using a transgenic mouse model of Alzheimer’s. The study was conducted by Charles River Laboratories. AL002 is a patented method using a mutant-peptide sensitized cell as a cell-based therapeutic vaccine that seeks to restore the ability of a patient’s immunological system to combat Alzheimer’s.
A five-dose GLP study with AL002-sensitized cells was completed using a transgenic (or genetically modified) mouse model of Alzheimer’s to investigate the tolerability of AL002. Single injections were administered on days 1, 30, 50, 70, and 90. The mice were evaluated for potential toxicity and reversibility of any findings at 75 and 90 days after dosing.
Histopathology results demonstrate that there was no indication of T-cell infiltration or meningoencephalitis suggesting that AL002 therapy is safe and tolerable as there were no adverse findings over a 90-day period and 90 days after the last dose. There were no treatment-related mortalities or reports of adverse effects on clinical observations, body weight parameters, organ weight parameters, clinical pathology parameters, gross pathology observations, or histopathologic observations during the main study or the recovery phase.
Modified cell therapies, especially DCs, may provide a safer and more patient-specific active immunization. Ex-vivo modification of DCs as a modality of treatment has been previously used in oncological therapeutics. It has been shown to be relatively safe and capable of engaging the immune system to attack the target tissues with success. Its use in Alzheimer’s therapeutics is relatively recent. We are proposing to conduct a first-in-human Phase I/II study of autologous DC, pulsed with a modified Aβ epitope. Preclinical work supports that it is associated with positive anti-inflammatory response and a decrease in brain amyloid contents. We anticipate submitting an IND to initiate a Phase I/II study for AL002 in September 2022.
A product can be designated as a breakthrough therapy if it is intended to treat a serious condition and preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over available therapy on a clinically significant endpoint(s). A drug that receives a breakthrough therapy designation is eligible for fast-track designation features, intensive guidance on an efficient drug development program and FDA organizational commitment involving senior managers. We believe that AL002 is positioned for a breakthrough therapy designation because of its positive effect on a pharmacodynamic biomarker (beta-amyloids) and potential for a clinically meaningful effect on Alzheimer’s. If we successfully acquire a breakthrough therapy designation for new drug approvals, we believe we can shorten the development timeline for AL002. However, we have neither received breakthrough therapy designation nor qualified for expedited development. Our product candidate may not qualify for breakthrough therapy designation; further, even if it does qualify for breakthrough therapy designation, it may not actually lead to faster development or expedited regulatory review and approval or necessarily increase the likelihood that it will receive FDA approval.
AL002 will require extensive clinical evaluation, regulatory review and approval, significant marketing efforts and substantial investment before it or any successors are likely to provide us with any revenue. As a result, if we do not successfully develop, achieve regulatory approval for and commercialize AL002, our long-term business plans will not be met, and we will be unable to generate the revenue we have forecast for the foreseeable future, if any. We do not anticipate that we will generate our maximum revenue for several years, or that we will achieve profitability for this therapeutic drug candidate until at least a few years after generating material revenue, if at all. If we are unable to generate revenue or raise substantial additional capital, we will not be able to pursue any expansion of our business or acquire additional intellectual property, we will not become profitable with this therapeutic drug candidate, and we will be unable to continue our operations at the currently planned pace, if at all.
Intellectual Property and Licensing Agreements
On June 2, 2018, we entered into two Standard Exclusive License Agreements with Sublicensing Terms for AL001 with the Licensor and its affiliate, the University of South Florida (the “AL001 License Agreements”), pursuant to which the Licensor granted us a royalty bearing exclusive worldwide licenses limited to the field of Alzheimer’s, under United States Patent Nos. (i) 9,840,521, entitled “Organic Anion Lithium Ionic Cocrystal Compounds and Compositions”, filed September 24, 2015 and granted December 12, 2017, and (ii) 9,603,869, entitled “Lithium Co-Crystals for Treatment of Neuropsychiatric Disorders”, filed May 21, 2016 and granted March 28, 2017.
| - 8 - |
The AL001 License Agreements require that we pay combined royalty payments of 4.5% on net sales of products developed from the licensed technology for AL001. We have already paid an initial license fee of $200,000 for AL001. As an additional licensing fee for the license of the AL001 technologies, the Licensor received 2,227,923 shares of our common stock. Minimum royalties for AL001 are $25,000 in 2023, $45,000 in 2024 and $70,000 in 2025 and every year thereafter, for the life of the AL001 License Agreements.
On May 1, 2016, we entered into a Standard Exclusive License Agreement with Sublicensing Terms for AL002 with the Licensor (the “AL002 License Agreement”), pursuant to which the Licensor granted us a royalty bearing exclusive worldwide license limited to the field of Alzheimer’s Immunotherapy and Diagnostics, under United States Patent No. 8,188,046, entitled “Amyloid Beta Peptides and Methods of Use”, filed April 7, 2009 and granted May 29, 2012.
The AL002 License Agreement requires us to pay royalty payments of 4% on net sales of products developed from the licensed technology for AL002. We have already paid an initial license fee of $200,000 for AL002. As an additional licensing fee for the license of AL002, the Licensor received 3,601,809 shares of our common stock. Minimum royalties for AL002 are $20,000 in 2022, $40,000 in 2023 and $50,000 in 2024 and every year thereafter, for the life of the AL002 License Agreement.
On June 10, 2020, we entered into two Standard Exclusive License Agreements with Sublicensing Terms for two additional indications of AL001 with the Licensor (the “June AL001 License Agreements”), pursuant to which the Licensor granted us a royalty bearing exclusive worldwide licenses limited to the fields of (i) neurodegenerative diseases excluding Alzheimer’s and (ii) psychiatric diseases and disorders.
The June AL001 License Agreements require us to pay royalty payments of 3% on net sales of products developed from the licensed technology for AL001 in those fields. We paid an initial license fee of $20,000 for the additional indications.
These license agreements have an indefinite term that continue until the later of the date no licensed patent under the applicable agreement remains a pending application or enforceable patent, the end date of any period of market exclusivity granted by a governmental regulatory body, or the date on which the licensee’s obligations to pay royalties expire under the applicable license agreement. Under our various license agreements, if we fail to meet a milestone by its specified date, Licensor may terminate the license agreement. The Licensor was also granted a preemptive right to acquire such shares or other equity securities that may be issued from time to time by us while the Licensor remains the owner of any equity securities of our company.
Additionally, we are required to pay milestone payments on the due dates to the Licensor for the license of the AL001 technologies and for the AL002 technology, as follows:
Original AL001 License:
| Payment | Due Date | Event | |||
| $ | 50,000 | * | Completed September 2019 | Pre-IND meeting | |
| $ | 65,000 | * | Completed June 2021 | IND application filing | |
| $ | 190,000 | * | Completed December 2021 | Upon first dosing of patient in a clinical trial | |
| $ | 500,000 | * | Completed March 2022 | Upon Completion of first clinical trial | |
| $ | 1,250,000 | 12 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 10,000,000 | 8 years from the effective date of the agreement | Upon FDA approval | ||
*Milestone met and completed
| - 9 - |
AL002 License:
| Payment | Due Date | Event | |||
| $ | 50,000 | * | Completed January 2022 | Upon IND application filing | |
| $ | 50,000 | 12 months from IND application filing date | Upon first dosing of patient in first Phase I clinical trial | ||
| $ | 175,000 | 12 months from first patient dosed in Phase I | Upon completion of first Phase I clinical trial | ||
| $ | 500,000 | 24 months from completion of first Phase I clinical trial | Upon completion of first Phase II clinical trial | ||
| $ | 1,000,000 | 12 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 10,000,000 | 7 years from the effective date of the agreement | Upon FDA Biologics License Application (“BLA”) approval | ||
* Milestone met and completed
Additional AL001 Licenses:
| Payment | Due Date | Event | |||
| $ | 50,000 | Upon IND application filing | IND application filing | ||
| $ | 150,000 | 12 months from IND filing date | Upon first dosing of patient in a clinical trial | ||
| $ | 400,000 | 12 months from first patient dosing | Upon Completion of first clinical trial | ||
| $ | 1,000,000 | 36 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 8,000,000 | 8 years from the effective date of the agreement | First commercial sale | ||
These license agreements have an indefinite term that continue until the later of the date no licensed patent under the applicable agreement remains a pending application or enforceable patent, the end date of any period of market exclusivity granted by a governmental regulatory body, or the date on which the licensee’s obligations to pay royalties expire under the applicable license agreement.
| - 10 - |
Market Opportunity
The Alzheimer’s Association estimates that the cost of caring for people with Alzheimer’s and other dementias will reach $321 billion in 2022, including $206 billion in Medicare and Medicaid payments, and that by 2050, these costs may rise as high as $1 trillion per year. Alzamend was formed to develop and commercialize patented intellectual property and treatments for Alzheimer’s, by funding it from preclinical through clinical trials and ultimately, if successful, make it available to the global market. Additionally, we are supporting ongoing research at the USF Health College of Medicine and plan to support others with first rights of refusal on technologies for treating terminal diseases.
In an article jointly issued on April 8, 2016, Allergan and Heptares cited currently significant unmet medical needs and a heavy economic burden caused by cognitive impairment and dementia across multiple diseases, noting that currently available drugs for the treatment of Alzheimer’s provide limited and transient effects on cognition. They cite projections of healthcare costs, including nursing home care, associated with Alzheimer’s and dementia (currently estimated to be in excess of $640 billion for North America, Western Europe, and Asia-Pacific), that are continuing to grow based on data from the World Health Organization, Alzheimer’s International, the National Institute of Mental Health and the Lewy Body Dementia Association.
This medical shortfall puts a spotlight on an urgent need for development of new therapies capable of treating the estimated more than 45 million people worldwide suffering from Alzheimer’s today, 6.2 million in North America, 7.5 million in Western Europe and 3.6 million in Asia-Pacific, a number expected to increase to more than 130 million by 2050. Alzheimer’s is the most common cause of dementia, estimated to be associated with some 60% to 70% of cases. An additional estimated 1.4 million patients in the United States suffer from Lewy body dementia. We believe that the potential marketplace for a commercialized therapy or treatment would be tremendously significant with large financial support available from numerous national and international pharmaceutical companies and various governments and worldwide agencies.
Industry Overview
Currently, Alzheimer’s is the sixth leading cause of death in the United States and, when extrapolated globally, the market for preventions, treatments and cures of this crippling disease is massive. Since 1990, life expectancy has increased by six years and the worldwide average continues to increase. With the increase in the mean age of the population in developed countries, the prevalence of deteriorating neurological diseases has also increased. According to the Alzheimer’s Association, in the United States alone, one of nine persons over the age of 65 have Alzheimer’s, with roughly 6.2 million Americans currently living with it. It is estimated that this number will grow to 13 million by 2050 barring the development of medical breakthroughs to prevent, slow or cure the disease. Many Alzheimer’s related associations believe the actual number of adults with Alzheimer’s may be much higher since current statistics do not take in account deaths from complications or from related diseases like pneumonia or heart attack. These death certificates only list the most immediate cause. The fastest growing age group in the United States is the “over 85” group within which one in three individuals have Alzheimer’s.
Although deaths from other major causes have decreased significantly, official records indicate that deaths from Alzheimer’s have increased significantly. Between 2000 and 2019, the number of deaths from Alzheimer’s as recorded on death certificates has more than doubled, increasing 145.2%, while the number of deaths from the number one cause of death (heart disease) decreased 7.3%.
Every 65 seconds, someone in the United States develops Alzheimer’s. Of the 10 most fatal diseases in the United States, Alzheimer’s is the only one with no cure, no known way of deceleration and no known means of prevention. Alzamend was formed to commercialize patented intellectual property in this space, by funding it from its present state through human clinical trials administered by the FDA and ultimately, if successful, potentially making it available to the global market.
Alzheimer’s
Alzheimer’s average annual incidence for individuals aged 65 to 74 was 0.4%. In individuals ages 75 to 84, the annual incidence was 3.2%, and for ages 85 and older (the “oldest-old”), the incidence was 7.6%. It is estimated that the cost of caring for people with Alzheimer’s and other dementias will increase from an estimated $305 billion in 2020 to a projected $1.1 trillion per year by 2050 with Medicare and Medicaid covering approximately 70% of such costs. Over 11 million Americans provide unpaid care for people with Alzheimer’s or other dementias. The Alzheimer’s Association estimates that, in 2021, caregivers to individuals with Alzheimer’s will provide 15.3 billion hours of care valued at $257 billion.
The cause and progression of Alzheimer’s are not well understood. Through May 2022, more than 3,793 clinical trials have been or are being conducted to find ways to treat the disease, but it is unknown if any of the tested treatments will work.
| - 11 - |
According to the Alzheimer’s Association, it is widely accepted that, with the increasing trend towards a longer lifespan coupled with the baby-boomer population approaching retirement, the incidence of Alzheimer’s is likely to double in the next 30 years. The exponential increase in the expected number of patients presenting with Alzheimer’s not only represents a major area of unmet medical need, but it also constitutes a significant market opportunity for diagnostics for this disease. Alzheimer’s biomarker sales in 2011 were reported at $1.5 billion but are expected have doubled in 2018 to over $3 billion. (BCC research 2013, “Advances in biomarker and monitoring diagnostics: Great markets, not so great health effects” by Bjørn Hofmann PhD and H. Gilbert Welch MD, MPH, 2017).
Current clinical research focuses on the early phases of the disease. However, to our knowledge, no accurate and convenient tools are available today for pre-dementia diagnosis of Alzheimer’s to support these efforts. Currently, Alzheimer’s is diagnosed using a process that combines cognition assessments with imaging and spinal-fluid tests. This diagnostic procedure may last for several months to a year and is usually initiated late in the disease development.
Several companies are focusing on blood as a test material. Typically, these companies employ a multi-assay strategy (multiple RNAs or proteins) combined with advanced statistical tools/algorithms to develop disease-specific diagnostic models.
Alzheimer’s Therapeutic Landscape
According to the Alzheimer’s Association, the following is a pictorial representation of the more recent published data encompassing the Alzheimer’s therapeutics landscape.
| - 12 - |
There are currently several experimental therapeutic agents for Alzheimer’s in various stages of development with clinical testing directed towards amyloid-beta, or Aβ, clearance, and inhibition of Tau protein aggregation or phosphorylated-Tau, or pTau, clearance. In June 2021, the FDA approved Biogen’s Alzheimer’s drug aducanumab, also known as Aduhelm, making it the first medication cleared by U.S. regulators to reduce amyloid plaques in people living with Alzheimer’s and the first new medication for the disease in nearly two decades. There were previously no drugs cleared by the FDA that can slow the mental decline from Alzheimer’s, which is the sixth-leading cause of death in the United States. The FDA approved Biogen’s Alzheimer’s drug Aduhelm, aimed at helping symptoms, not actually slowing the disease itself. Recent clinical failures involving Aβ clearance highlight the incomplete understanding of the pathological processes in Alzheimer’s and clearly demonstrate the need for novel strategies to fight the disease.
Clinical Management
We have retained Rio Pharmaceutical Services and TAMM Net, Inc., to lead, develop and manage our preclinical and clinical efforts, extending from the current status of each product candidate through the exit or commercialization of the technologies that we have licensed. We may retain experienced Canadian and European Union consulting firms to commercialize these same technologies for those geographic markets.
Manufacturing
Currently, we do not have in-house manufacturing capabilities. We have outsourced and expect to continue to outsource the manufacturing of our products to third party contractors, with special capabilities to manufacture chemical drugs and biologic drug candidates for submission and clinical testing under FDA guidelines and, for AL001, have received Good Manufacturing Practices, or GMP, material manufactured for clinical trial. There are several sources of manufacturing available once a therapy or treatment can achieve Phase II study as identified in a publication by Pharma.org released in 2013 (http://www.phrma.org/sites/default/files/Alzheimer’s%202013.pdf).
Distribution and Marketing
We intend to develop AL001 and AL002 through successive de-risking milestones towards regulatory approval and seek marketing approval of AL001 and AL002 or enter into partnering transactions with biopharmaceutical companies seeking to strategically fortify pipelines and, in turn, receiving funding for the costly later-stage clinical development required to achieve successful commercialization. We do not anticipate selling products directly into the marketplace, though we may do so depending on market conditions. Our focus is to strategically effect partnering transactions which will provide distribution and marketing capabilities to sell products into the marketplace.
Government Regulation
Clinical trials, the pharmaceutical approval process, and the marketing of pharmaceutical products, are intensively regulated in the United States and in all major foreign countries.
Human Health Product Regulation in the United States
In the United States, the FDA regulates pharmaceuticals under the Federal Food, Drug, and Cosmetic Act and related regulations promulgated thereunder. Pharmaceuticals are also subject to other federal, state, and local statutes and regulations. Failure to comply with applicable U.S. regulatory requirements at any time during the product development process, approval process or after approval may subject an applicant to administrative or judicial sanctions. These sanctions could include the imposition by the FDA of an Institutional Review Board, or IRB, a clinical hold on trials, a refusal to approve pending applications, withdrawal of an approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency or judicial enforcement action could have a material adverse effect on us.
The FDA and comparable regulatory agencies in state and local jurisdictions impose substantial requirements upon the clinical development, manufacturing and marketing of pharmaceutical products. These agencies and other federal, state and local entities regulate research and development activities and the testing, manufacture, quality control, safety, effectiveness, labeling, storage, distribution, record keeping, approval, advertising and promotion of our products.
The FDA’s policies may change, and additional government regulations may be enacted that could prevent or delay regulatory approval of new disease indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative or administrative action, either in the United States or elsewhere.
| - 13 - |
Marketing Approval
The process required by the FDA before human health care pharmaceuticals may be marketed in the U.S. generally involves the following:
| • | nonclinical laboratory and, at times, animal tests; |
| • | adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use or uses; |
| • | pre-approval inspection of manufacturing facilities and clinical trial sites; and |
| • | FDA approval of an NDA or BLA, which must occur before a drug or biologic product can be marketed or sold. |
We will need to successfully complete sufficient clinical trials in order to be in a position to submit a BLA or NDA to the FDA. We will reach agreement with the FDA on the proposed protocols for our future clinical trials in the U.S. A separate submission to the FDA must be made for each successive clinical trial to be conducted during product development. Further, an independent IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site, and an informed consent must also be obtained from each study subject. Regulatory authorities, a data safety monitoring board or the sponsor may each suspend or terminate a clinical trial at any time on numerous grounds.
For purposes of BLA or NDA approval for human health products, human clinical trials are typically conducted in phases that may overlap.
| • | Phase I. The drug is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| • | Phase II. This phase involves trials in a limited subject population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. Phase II studies may be sub-categorized into Phase IIA studies which are smaller, pilot studies to evaluate limited drug exposure and efficacy signals, and Phase IIB studies, which are larger studies testing both safety and efficacy more rigorously. |
| • | Phase III. This phase involves trials undertaken to further evaluate dosage, clinical efficacy and safety in an expanded subject population, often at geographically dispersed clinical trial sites. These trials are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. |
All of these trials must be conducted in accordance with Good Clinical Practice (“GCP”), requirements in order for the data to be considered reliable for regulatory purposes.
New Drug and Biologics License Applications
In order to obtain approval to market a pharmaceutical in the United States, a marketing application must be submitted to the FDA that provides data establishing to the FDA’s satisfaction the safety and effectiveness of the investigational drug for the proposed indication. Each NDA or BLA submission requires a substantial user fee payment unless a waiver or exemption applies (such as with the Orphan Drug Designation discussed below). For fiscal year 2021, the FDA set the application fee at $2,875,842 for new drug applications that require clinical data. The manufacturer and/or sponsor of certain drugs approved under an NDA or BLA is also subject to annual prescription drug program fees, currently set at $336,432 per product for fiscal year 2021. These fees are typically increased annually. The NDA or BLA includes all relevant data available from pertinent non-clinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls and proposed labeling, among other things. Data can come from company-sponsored clinical trials intended to test the safety and effectiveness of the use of a product, or from a number of alternative sources, including studies initiated by investigators.
| - 14 - |
The FDA will initially review the NDA or BLA for completeness before it accepts it for filing. The FDA has 60 days from its receipt of an NDA or BLA to determine whether the application will be accepted for filing based on the agency’s threshold determination that the application is sufficiently complete to permit substantive review. After the NDA or BLA submission is accepted for filing, the FDA reviews the NDA or BLA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with current GMP, or cGMP, to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug products or drug products that present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and, if so, under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it typically considers such recommendations carefully when making decisions.
Based on pivotal Phase III trial results submitted in an NDA or BLA, upon the request of an applicant, the FDA may grant a “Priority Review” designation to a product, which sets the target date for FDA action on the application at six to eight months, rather than the standard ten to 12 months. The FDA can extend these reviews by three months. Priority Review is given where preliminary estimates indicate that a product, if approved, has the potential to provide a significant improvement compared to marketed products or offers a therapy where no satisfactory alternative therapy exists. Priority Review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
After the FDA completes its initial review of an NDA or BLA, it will communicate to the sponsor that the application for the drug will either be approved, or it will issue a complete response letter to communicate that the NDA or BLA will not be approved in its current form and inform the sponsor of changes that must be made or additional clinical, nonclinical or manufacturing data that must be received before the application can be approved, with no implication regarding the ultimate approvability of the application.
Before approving an NDA or BLA, the FDA will inspect the facilities at which the product is manufactured, even if such facilities are located overseas. The FDA will not approve the product unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications.
Additionally, before approving an NDA or BLA, the FDA may inspect one or more clinical sites and manufacturing sites to assure compliance with GCP and GMP. If the FDA determines that any of the application, manufacturing process or manufacturing facilities is not acceptable, it typically will outline the deficiencies and often will request additional testing or information. This may significantly delay further review of the application. If the FDA finds that a clinical site did not conduct the clinical trial in accordance with GCP, the FDA may determine that the data generated by the clinical site should be excluded from the primary efficacy analyses provided in the NDA or BLA. Additionally, the FDA may identify deficiencies in the manufacturing process and require changes prior to approval. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
The testing and approval process for a drug requires substantial time, effort and financial resources, and this process may take up to several years to complete. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products.
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase IV studies may be made a condition that must be satisfied for continuing drug approval. The results of Phase IV studies can confirm the effectiveness of a product candidate and can provide important safety information. In addition, the FDA has express statutory authority to require sponsors to conduct post-market studies to specifically address safety issues identified by the agency. Any approvals that we may ultimately receive could be withdrawn if required post-marketing trials or analyses do not meet the FDA requirements, which would materially harm the commercial prospects for AL001 or AL002.
The FDA also has authority to require a Risk Evaluation and Mitigation Strategy (“REMS”), from manufacturers to ensure that the benefits of a drug or biological product outweigh its risks. A sponsor may also voluntarily propose a REMS as part of the NDA or BLA submission. The need for a REMS is determined as part of the review of the NDA or BLA. Based on statutory standards, elements of a REMS may include “dear doctor letters,” a medication guide, more elaborate targeted educational programs, and in some cases restrictions on distribution. These elements are negotiated as part of the NDA or BLA approval, and in some cases if consensus is not obtained until after the Prescription Drug User Fee Act review cycle, the approval date may be delayed. Once adopted, a REMS is subject to periodic assessment and modification.
Even if AL001 or AL002 receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, or in the form of onerous risk management plans, restrictions on distribution, or post-marketing study requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Any delay in obtaining, or failure to obtain, regulatory approval for AL001 or AL002, or obtaining approval only for significantly limited use, would harm our business. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
| - 15 - |
Section 505(b)(2) New Drug Applications
Companies may also consider seeking FDA approval through the Section 505(b)(2) NDA process if their product candidates are similar to previously approved drugs but differ in dosage form, strength, route of administration, formulation or indication. Section 505(b)(2) of the Food, Drug, and Cosmetic Act was enacted as part of the Drug Price Competition and Patent Term Restoration Act of 1984 and is also known as the Hatch-Waxman Amendments. The purpose of Section 505(b)(2) is to allow companies to avoid duplicative testing by allowing applicants to utilize data from previous clinical and non-clinical studies in the current NDA submission, when pertinent. The 505(b)(2) application process requires, among other things, the submission of data from studies demonstrating the product’s safety and efficacy for the new indication.
The Hatch-Waxman Amendments permit companies to rely upon not only certain published nonclinical or clinical studies conducted for an approved product, but also the FDA’s conclusions from a prior review of the studies. Additionally, the FDA may require companies to perform further studies to support changes from the approved product. After completion of the review, the FDA may approve the new product for all or some of the labeled indications for which the reference product has been approved, as well as for any new indication supported by the NDA. While references to nonclinical and clinical data not created by the applicant or for which the applicant does not have a right of reference are allowed, the applicant must still submit data related to the manufacturing and quality of the product candidate, such as information about the development, process, stability, qualification and validation.
If a company chooses to rely on the FDA’s conclusions regarding studies conducted for an already approved product, the company is required to provide a certification statement for any patents listed for the approved product in the FDA’s Orange Book publication. Specifically, the applicant must certify that: (i) the required patent information has not been filed; (ii) the listed patent has expired; (iii) the listed patent has not expired but will expire on a particular date and approval is sought after patent expiration; or (iv) the listed patent is invalid or will not be infringed by the new product. The FDA will also not approve a Section 505(b)(2) until any non-patent exclusivity period for the reference product has expired, such as the exclusivity granted for obtaining approval of a new chemical entity.
Disclosure of Clinical Trial Information
Sponsors of clinical trials of certain FDA-regulated products, including prescription drugs, are required to register and disclose certain clinical trial information on a public website maintained by the U.S. National Institutes of Health. Information related to the product, patient population, phase of investigation, study sites and investigator, and other aspects of the clinical trial is made public as part of the registration. Sponsors are also obligated to disclose the results of these trials after completion. Disclosure of the results of these trials can be delayed until the product or new indication being studied has been approved. Competitors may use this publicly available information to gain knowledge regarding the design and progress of our development programs.
The Drug Price Competition and Patent Term Restoration Act
The Drug Price Competition and Patent Term Restoration Act, also known as the Hatch-Waxman Amendments, requires pharmaceutical companies to divulge certain information regarding their products which has the effect of making it easier for other companies to manufacture generic drugs to compete with those products.
Patent Term Extension. After receipt of an NDA or BLA approval, owners of relevant drug patents may apply for a patent extension of up to five years. The permissible patent term extension is calculated as half of the drug’s testing phase, that is, the time between IND submission and NDA or BLA submission, and all of the review phase, or the time between either NDA or BLA submission and approval up to a maximum of five years. The time can be shortened if FDA determines that the applicant did not pursue approval with due diligence. The total patent term after the extension may not exceed 14 years.
For patents that might expire during the application phase, the patent owner may request an interim patent extension. An interim patent extension increases the patent term by one year and may be renewed up to four times. For each interim patent extension granted, the post-approval patent extension is reduced by one year. The director of the U.S. Patent and Trademark Office, or USPTO, must determine that approval of the drug covered by the patent for which a patent extension is being sought is likely. Interim patent extensions are not available for a drug for which an NDA or BLA has not been submitted.
Environmental Regulations. The U.S. generally requires an environmental assessment, which discusses a company’s proposed action, possible alternatives to the action, and whether the further analysis of an environmental impact statement is necessary. Certain exemptions are available from the requirement to perform an environmental assessment and an environmental impact statement. Once an exemption is claimed, a company must state to the FDA that no extraordinary circumstances exist that may significantly affect the environment. We may claim an exemption, under the category for biologic products, from the requirement to provide an environmental assessment and an environmental impact statement for AL001 or AL002 and further state to the FDA that, to our knowledge, no extraordinary circumstances exist that would significantly affect the environment.
| - 16 - |
FDA Post-Approval Requirements
Following the approval of an NDA or BLA, the FDA continues to require adverse event reporting and submission of periodic reports. The FDA also may require post-marketing testing, known as Phase IV testing, REMS, and surveillance to monitor the effects of an approved product, or the FDA may place conditions on an approval that could restrict the distribution or use of the product. In addition, quality control, drug manufacture, packaging, and labeling procedures must continue to conform to cGMP after approval. Drug manufacturers and certain of their subcontractors are required to register their establishments with FDA and certain state agencies. Registration with the FDA subjects entities to periodic unannounced inspections by the FDA, during which the agency inspects manufacturing facilities to assess compliance with cGMP. Accordingly, manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMP. Regulatory authorities may withdraw product approvals or request product recalls if a manufacturer fails to comply with regulatory standards, if it encounters problems following initial marketing or if previously unrecognized problems are subsequently discovered.
Patient Protection and Affordable Care Act
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, or the ACA, which includes measures that have significantly changed the way health care is financed by both governmental and private insurers, became law in the U.S. The ACA is a sweeping measure intended to expand health care coverage within the U.S., primarily through the imposition of health insurance mandates on employers and individuals and expansion of the Medicaid program. The ACA has significantly impacted the pharmaceutical industry. The ACA requires discounts under the Medicare drug benefit program and increased rebates on drugs covered by Medicaid. In addition, the ACA imposes an annual fee, which increases annually, on sales by branded pharmaceutical manufacturers. There have been significant ongoing judicial, administrative, executive and legislative efforts to modify, amend or eliminate the ACA. For example, a Texas U.S. District Court Judge ruled that the ACA is unconstitutional in its entirety because the “individual mandate” was repealed by Congress. The case has been appealed to the U.S. Supreme Court and is awaiting a ruling. At this time, the financial impact of these discounts, increased rebates and fees and the other provisions of the ACA on our business are unclear. However, the fees, discounts and other provisions of this law are expected to have a significant negative effect on the profitability of pharmaceuticals.
Human Health Product Regulation in the European Union
In addition to domestic regulations, we may eventually be subject, either directly or through our distribution partners, to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products, if approved.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in non-U.S. countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the U.S. have a process that requires the submission of a clinical trial application prior to the commencement of human clinical trials. In Europe, for example, a Clinical Trial Application (“CTA”) must be submitted to the competent national health authority and to independent ethics committees in each country in which a company intends to conduct clinical trials. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed in that country.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country, even though there is already some degree of legal harmonization in the EU Member States resulting from the national implementation of underlying EU legislation. In all cases, the clinical trials are conducted in accordance with GCP and other applicable regulatory requirements.
To obtain regulatory approval of an investigational drug under European Union regulatory systems, we will be required to submit a marketing authorization application. This application is similar to the BLA in the United States, with the exception of, among other things, country-specific document requirements. Drugs can be authorized in the European Union by using (i) the centralized authorization procedure, (ii) the mutual recognition procedure, (iii) the decentralized procedure or (iv) the national authorization procedure.
The European Medicines Agency (“EMA”) implemented the centralized procedure for the approval of human drugs to facilitate marketing authorizations that are valid throughout the EU. This procedure results in a single marketing authorization granted by the European Commission that is valid across the EU, as well as in Iceland, Liechtenstein and Norway, at times referred to as the European Economic Area. The centralized procedure is compulsory for human drugs that: (i) are derived from biotechnology processes, such as genetic engineering; (ii) contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative diseases, autoimmune and other immune dysfunctions and viral diseases; (iii) are officially designated orphan drugs; and (iv) constitute advanced-therapy medicines, such as gene-therapy, somatic cell-therapy or tissue-engineered medicines. The centralized procedure may at the request of the applicant also be used for human drugs that do not fall within the above mentioned categories if the human drug (a) contains a new active substance which, on the date of entry into force of Regulation (EC) No. 726/2004, was not authorized in the European Economic Area; or (b) the applicant shows that the medicinal product constitutes a significant therapeutic, scientific or technical innovation or that the granting of authorization in the centralized procedure is in the interests of patients at European Economic Area level.
| - 17 - |
Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of a Marketing Authorization Application by the EMA is 210 days, though the date count stops whenever the Committee for Medicinal Products for Human Use (“CHMP”) asks the applicant for additional written or oral information, with adoption of the actual marketing authorization by the European Commission thereafter. Accelerated evaluation might be granted by the CHMP in exceptional cases, as when a medicinal product is expected to be of a major public health interest from the point of view of therapeutic innovation, defined by three cumulative criteria: (i) the seriousness of the disease to be treated; (ii) the absence of an appropriate alternative therapeutic approach; and (iii) anticipation of exceptional high therapeutic benefit. In this circumstance, EMA ensures that the evaluation for the opinion of the CHMP is completed within 150 days and the opinion issued thereafter.
The Mutual Recognition Procedure (“MRP”) for the approval of human drugs is an alternative approach to facilitate individual national marketing authorizations within the European Union. Essentially, the MRP may be applied for all human drugs for which the centralized procedure is not obligatory. The MRP is applicable to the majority of conventional medicinal products and is based on the principle of recognition of an already existing national marketing authorization by one or more EU Member States.
The principal characteristic of the MRP is that the procedure builds on an already existing marketing authorization in an EU Member State that is used as reference in order to obtain marketing authorizations in other Member States. In the MRP, a marketing authorization for a drug already exists in one or more EU Member States and subsequently marketing authorization applications are made in other EU Member States by referring to the initial marketing authorization. The EU Member State in which the marketing authorization was first granted will then act as the reference EU Member State. The EU Member States where the marketing authorization is subsequently applied for act as concerned EU Member States.
The MRP is based on the principle of the mutual recognition by EU Member States of their respective national marketing authorizations. Based on a marketing authorization in the reference EU Member State, the applicant may apply for marketing authorizations in other EU Member States. In such case, the reference EU Member State will update its existing assessment report about the drug in 90 days. After the assessment is completed, copies of the report are sent to all EU Member States, together with the approved summary of product characteristics, labeling and package leaflet. The concerned EU Member States then have 90 days to recognize the decision of the referenced EU Member State and the summary of product characteristics, labeling and package leaflet. National marketing authorizations will be granted within 30 days after acknowledgement of the agreement.
If any EU Member State refuses to recognize the marketing authorization by the reference EU Member State on the grounds of potential serious risk to public health, the issue will be referred to a coordination group. Within 60 days, EU Member States will, within the coordination group, make all efforts to reach a consensus. If this fails, the procedure is submitted to an EMA scientific committee for arbitration. The opinion of this EMA Committee is then forwarded to the Commission, for the start of the decision-making process. As in the centralized procedure, this process entails consulting various European Commission Directorates General and the Standing Committee on Human Medicinal Products.
Human Health Product Regulation in the Rest of World
For countries outside of the EU, such as Canada, countries in Eastern Europe or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and the other applicable regulatory requirements. If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension of clinical trials, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Other Regulatory Considerations
Labeling, Marketing and Promotion. Once an NDA or BLA is approved, or just before approval, a product will be subject to certain marketing and promotional requirements. For instance, the FDA closely regulates the post-approval marketing and promotion of pharmaceuticals, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored scientific and educational activities and promotional activities on the internet and elsewhere.
While doctors are free to prescribe any pharmaceutical approved by the FDA for any use, a company can only make claims relating to the safety and efficacy of a pharmaceutical that are consistent with the FDA approval, and is only allowed to actively market a pharmaceutical for the particular indication approved by the FDA. Changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or BLA or NDA/BLA supplement before the change can be implemented. A BLA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the same procedures and actions in reviewing supplements as it does in reviewing NDAs.
| - 18 - |
In addition, any claims we make for our products in advertising or promotion must be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising, injunctions and potential civil and criminal penalties. Government regulators recently have increased their scrutiny of the promotion and marketing of pharmaceuticals.
Anti-Kickback and False Claims Laws. In the United States, we are subject to complex laws and regulations pertaining to health care “fraud and abuse,” including, but not limited to, the federal Anti-Kickback Statute, the federal False Claims Act, state false claims acts and anti-kickback statutes, and other state and federal laws and regulations. The Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf) to knowingly and willfully solicit, receive, offer, or pay any remuneration that is intended to induce the referral of business, including the purchase, order, or prescription of a particular pharmaceutical, for which payment may be made under a federal health care program, such as Medicare or Medicaid.
The federal False Claims Act prohibits anyone from knowingly presenting, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services, including pharmaceuticals, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services.
Many states have similar anti-kickback or false claims statutes that can be even broader than their federal counterparts. There is also an increasing number of state laws that require manufacturers to make reports to states on pricing and marketing information. Many of these laws contain ambiguities as to what is required to comply with the laws. In addition, a federal law known as the Physician Payments Sunshine Act requires pharmaceutical manufacturers to track and report to the federal government certain payments and other transfers of value made to physicians and teaching hospitals and to disclose any physician ownership in the previous calendar year. The data is published annually in a publicly searchable database. These laws may affect our sales, marketing, and other promotional activities by imposing administrative and compliance burdens on us. In addition, given the lack of clarity with respect to these laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent state, and soon federal, authorities.
Other Health Care Laws and Compliance Requirements. In the United States, our activities are potentially subject to regulation by various federal, state and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services (formerly the Health Care Financing Administration), other divisions of the U.S. Department of Health and Human Services (e.g., its Office of Inspector General), the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments. For example, sales, marketing and scientific/ educational grant programs must comply with the anti-fraud and abuse provisions of the Social Security Act, the False Claims Act, the privacy provisions of the Health Insurance Portability and Accountability Act, and similar state laws, each as amended. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and the Veterans Health Care Act of 1992, or VHCA, each as amended, among others. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements will apply. Under the VHCA, drug companies are required to offer certain drugs at a reduced price to a number of federal agencies including U.S. Department of Veteran Affairs and U.S. Department of Defense, the Public Health Service and certain private Public Health Service designated entities in order to participate in other federal funding programs including Medicare and Medicaid. Legislative changes also require that discounted prices be offered for certain U.S. Department of Defense purchases for its TRICARE program via a rebate system. Participation under the VHCA requires submission of pricing data and calculation of discounts and rebates pursuant to complex statutory formulas, as well as the entry into government procurement contracts governed by the Federal Acquisition Regulations.
In order to distribute products commercially, we must comply with state laws that require the registration of manufacturers and wholesale distributors of pharmaceutical products in a state, including, in certain states, manufacturers and distributors that ship products into the state even if such manufacturers or distributors have no place of business within the state. Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities or register their sales representatives. Other legislation has been enacted in certain states prohibiting pharmacies and other health care entities from providing certain physician prescribing data to pharmaceutical companies for use in sales and marketing and prohibiting certain other sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection, unfair competition and other laws and regulations.
| - 19 - |
Our Intellectual Property
We are able to protect our technology from unauthorized use by third parties only to the extent that it is covered by valid and enforceable patents or is effectively maintained as a trade secret or is protected by confidentiality agreements. Accordingly, patents or other proprietary rights are an essential element of our business. Currently, we do not own a patent, although we do possess a license for an immunotherapy technology and two licenses for a lithium, salicylate and proline cocrystal technology from the University of South Florida.
Patents extend for varying periods according to the date of patent filing or grant and the legal term of patents in the various countries where patent protection is obtained. The actual protection afforded by a patent, which can vary from country to country, depending on the type of patent, the scope of its coverage and the availability of legal remedies in the country.
A summary of the licensed patents is as follows:
| Title of Patent | Patent Type | Therapeutic Drug |
Date Filed |
Date Issued |
Expiration Date |
Patent # |
| Lithium Cocrystals and an Additional Neuropsychiatric Agent for Treatment of Neuropsychiatric Disorders |
Method of Use |
AL001 (LISPRO) |
05/21/2016 | 03/28/2017 | 05/21/2036 | 9,603,869 |
| Organic Anion Lithium Ionic Cocrystal Compounds and Compositions | Composition of Matter |
AL001 (LISPRO) |
04/18/2014 | 12/12/2017 | 04/18/2034 | 9,840,521 |
| Amyloid Beta Peptides and Methods of Use | Composition of Matter |
AL002 (E22W) |
10/12/2007 | 05/29/2012 | 02/12/2028 | 8,188,046 |
While trade secret protection is an essential element of our business and we take security measures to protect our proprietary information and trade secrets, there can be no assurance that our unpatented proprietary technology will afford us significant commercial protection. We seek to protect our trade secrets by entering into confidentiality agreements with third parties, employees and consultants. However, it is possible that these agreements may be breached or invalidated, and if so, there may not be an adequate corrective remedy available. Accordingly, we cannot ensure that our employees, consultants or any third parties will not breach the confidentiality provisions in our contracts, infringe or misappropriate our trade secrets and other proprietary rights or that measures we take to protect our proprietary rights will be adequate.
In the future, third parties may file claims asserting that our technologies or products infringe on their intellectual property. We cannot predict whether third parties will assert such claims against us or against the licensors of technology licensed to us, or whether those claims will harm our business. If we are forced to defend ourselves against such claims, whether they are with or without merit and whether they are resolved in favor of, or against, our licensors or ourselves, we may face costly litigation and the diversion of our management’s attention and resources. As a result of such disputes, we may have to develop costly non-infringing technology or enter into licensing agreements. These agreements, if necessary, may be unavailable on terms acceptable to us, or at all.
We currently have four trademarks registered with the USPTO that include our corporate name, Alzamend Neuro, two for our corporate slogan and one for our trade name.
Our Competition
Our industry is highly competitive and subject to rapid and significant technological change. While we have some, albeit limited, development experience and scientific knowledge, we will face competition from both large and small pharmaceutical and biotechnology companies, including specialty pharmaceutical companies and generic drug companies, as well as academic institutions, government agencies and research institutions, among others.
Our competition will be determined in part by the potential indications for which our products are developed and ultimately approved by regulatory authorities. It is likely that the timing of market introductions of some of our potential products or our competitors’ products will be an important competitive factor. Accordingly, the speed with which we can develop our products, conduct preclinical studies and clinical trials to obtain approval and manufacture or obtain supplies of commercial quantities of any approved products should also be important competitive factors. We expect that competition among products approved for sale will be based on additional factors such as product efficacy, safety, reliability, availability, price and patent position.
Employees and Human Capital Resources
As of April 30, 2022, we have four full-time employees (Stephan Jackman, our Chief Executive Officer, Lien T. Escalona, our Chief Financial Officer, Bradford Sullivan, our Director of Investor Relations, and Kraingsak Roajphlastien, our Senior Operations Manager) and four part-time employees. We also utilize independent consultants to assist us in our medical research and development projects.
| - 20 - |
Henry C.W. Nisser, our Executive Vice President and General Counsel, Kenneth S. Cragun, our Senior Vice President of Finance, David Katzoff, our Chief Operating Officer and James M. Turner, our Deputy General Counsel, work for us on a part-time basis.
Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and new employees, advisors and consultants. The principal purposes of our equity and cash incentive plans are to attract, retain and reward personnel through the granting of stock-based and cash-based compensation awards, in order to increase stockholder value and the success of our company by motivating such individuals to perform to the best of their abilities and achieve our objectives.
Scientific Advisory Board
Our scientific advisory board of leading researchers in the neurodegenerative and neuropathology fields presently consists of Dr. Thomas M. Wisniewski, Dr. Eric McDade and Dr. Terri Hunter.
Thomas M. Wisniewski, MD is a board-certified neurologist and neuropathologist and is the Director of the NYU Pearl I. Barlow Center for Memory Evaluation and Treatment. He operates an active research laboratory focusing on neurodegenerative disorders with a particular focus on the mechanisms that drive amyloid deposition in Alzheimer’s and prion diseases. This work has led to more than 300 peer-reviewed publications, 28 issued patents, and continuous funding from the NIH for over 30 years. Dr. Wisniewski’s career has been dedicated to researching and developing treatments for numerous conditions including Alzheimer’s, mild cognitive impairment, Lewy body dementia, frontotemporal dementia, prion disease, Jakob-Creutzfeldt disease, multiple system atrophy and memory loss. This has led him to receive numerous awards, honors and recognitions including being elected as a Distinguished Fellow in 2014, receiving the 2009 Prion Prize, the Alzheimer’s Association Zenith Award in 2002 and being recognized every year by “Best Doctors in America” since 2008. Dr. Wisniewski has been an Associate Editor for the Journal of Alzheimer’s Disease and Chief Editor of Frontiers in Aging Neuroscience since 2018. Dr. Wisniewski earned his M.D. degree at King’s College London GKT School of Medical Education and completed his residencies and chief residencies in neurology and neuropathology at NYU School of Medicine and New York-Presbyterian/Columbia University Medical Center, respectively.
Eric M. McDade, DO is a board-certified cognitive neurologist who has focused his activities on the evaluation of those with dementia syndromes and on developing a clinical research program that focuses on using brain imaging and cerebrospinal fluid markers to identify those at risk for Alzheimer’s. Currently, Dr. McDade is leveraging his clinical expertise to develop a cross-disciplinary team that combines neuroimaging, clinical evaluations and basic science to better explore and translate work in the use of imaging and fluid biomarkers to better understand the timing and relationship between measures of disease risk and progression. The goal of this work is to identify better measures and target for interventions and prevention for Alzheimer’s and has led to more than 76 peer-reviewed publications and continuous funding from the NIH for over 10 years. Additionally, Dr. McDade is the Associate Director of the Dominantly Inherited Alzheimer Network Trials Unit (“DIAN-TU”). The DIAN-TU is a global network of families at risk for dominantly inherited Alzheimer’s, a genetic form of Alzheimer’s and is pioneering prevention trials for this young-onset form of Alzheimer’s. Dr. McDade earned his doctorate at Chicago College of Osteopathic Medicine and a B.A. degree in Psychology from Canisius College. Dr. McDade completed an internship at the University of Illinois College of Medicine in Chicago and his residency at the University of Maryland. Dr. McDade received his certification of Neurology from the American Board of Psychiatry and Neurology and Behavioral Neurology from the United Council of Neurologic Subspecialties.
Terri Hunter, PhD is a Technology Transfer Specialist at the United States Department of Veterans Affairs (USDVA). Dr. Hunter joined the USDVA in September 2020 and is responsible for managing life science technologies from initial disclosure through licensing and the maintenance of the license. Prior to joining the USDVA, Dr. Hunter worked as a senior licensing manager in the technology transfer Office, patents & licensing at the University of South Florida for 9 years (2010 to 2020). From 2003 to 2010, Dr. Hunter worked as a Research Scientist at Moffitt Cancer Center in Tampa, Florida. At Moffitt Dr. Hunter performed translational research focused on cancer vaccines and combination therapies for cancer. She has also served as a DNA analyst/expert witness for the Florida Department of Law Enforcement. Her post-doctoral training was conducted at St. Jude Children’s Research Hospital in Memphis, Tennessee form 1998-2000. She received a B.S. in Biology from Palm Beach Atlantic University in 1994, a M.S. in Medical Sciences from the University of South Florida, College of Medicine in 1997, and a Ph.D. in Medical Sciences from the University of South Florida, College of Medicine (1998, Medical Microbiology and Immunology Program). Dr. Hunter’s research interests included microbial genetics, DNA analysis, cell signaling, immunobiology of cancer, gene-modified tumor cell vaccine research: specifically pre-clinical and clinical research and combination biologic and pharmacologic treatments for cancer.
We entered into consulting agreements with Drs. Wisniewski and McDade on May 1, 2019 and Dr. Hunter on April 4, 2022. The annual cash compensation under the consulting agreements consists of $12,000 per scientific advisory board member. Drs. Wisniewski and McDade were awarded stock options to purchase 50,000 shares at $1.00 per share with a two-year term, vesting over two years. Dr. Hunter was awarded stock options to purchase 50,000 shares at $2.42 per share with a two-year term, vesting over two years.
| - 21 - |
| ITEM 1A. | RISK FACTORS |
Investing in our common stock involves a high degree of risk. You should carefully consider the risks described below, as well as the other information in this Annual Report, including our financial statements and the related notes and the section of this Annual Report titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” before deciding whether to invest in our common stock. The occurrence of any of the events or developments described below could harm our business, financial condition, results of operations and growth prospects. In such an event, the market price of our common stock could decline and you may lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also impair our business operations.
Risks Related to Our Company, Early Clinical-Stage of Development and Financial Condition
We are at an early clinical-stage of development and currently have no source of near-term revenue and may never become profitable.
We are an early clinical-stage biopharmaceutical company. We have recently initiated clinical trials for our AL001 and AL002 programs. To date, we have not initiated or completed a pivotal clinical trial, obtained marketing approval for any product candidates, manufactured a commercial scale product or arranged for a third party to do so on our behalf, or conducted sales and marketing activities necessary for successful product commercialization. Our ability to generate revenue depends heavily on, among other developments:
| • | demonstration to the satisfaction of the FDA and comparable regulatory bodies that AL001 and AL002 are safe and effective in future clinical trials; |
| • | our ability to seek and obtain regulatory approvals, including with respect to the indications we are seeking; |
| • | if approved by the FDA, successful manufacture and commercialization of AL001 and AL002; and |
| • | market acceptance of AL001 and AL002. |
We only have two product candidates, AL001 and AL002, which will require extensive clinical evaluation, regulatory review and approval, significant marketing efforts and substantial investment before either or both of them, and any respective successors, will provide us with any revenue. As a result, if we do not successfully develop, achieve regulatory approval for and commercialize AL001 or AL002, we will be unable to generate any revenue for many years, if at all. We do not anticipate that we will generate revenue for a few years, at the earliest, or that we will achieve profitability for at least several years after generating material revenue, if at all. If we are unable to generate revenue, we will not become profitable, and we may be unable to continue our operations.
We have a limited operating history on which to judge our business prospects and management.
We were incorporated in February 2016 and commenced operations shortly thereafter. We have a limited operating history upon which to base an evaluation of our business and prospects. Operating results for future periods are subject to numerous uncertainties and we cannot assure you that we will achieve or sustain profitability. Our prospects must be considered in light of the risks encountered by companies in the early stage of development, particularly companies in new and rapidly evolving markets. Future operating results will depend upon many factors, including our success in attracting and retaining motivated and qualified personnel, our ability to establish short term credit lines or obtain financing from other sources, our ability to develop and market new products or control costs, and general economic conditions. We cannot assure you that we will successfully address any of these contingencies.
We will need, but may be unable to obtain, funding on satisfactory terms, which could dilute our stockholders and investors, and/or impose burdensome financial restrictions on our business.
We have relied upon cash from financing activities and in the future, we hope to rely on revenues generated from operations to fund all of the cash requirements of our activities. However, it is extremely unlikely that we will be able to generate any significant cash from our operating activities in the foreseeable future. Future financings may not be available on a timely basis, in sufficient amounts or on terms acceptable to us, if at all. Any debt financing or other financing of securities senior to our common stock will likely include financial and other covenants that will restrict our flexibility. Any failure to comply with these covenants may cause an event of default and acceleration of the obligation to pay the debt, which would have a material adverse effect on our business, prospects, financial condition and results of operations and we could lose our existing sources of funding and impair our ability to secure new sources of funding. There can be no assurance that we will be able to generate any further investor interest in our securities or other types of funding, in which case you would likely lose the entirety of your investment in us.
| - 22 - |
Risks Related to Our Product Candidates
We have both operational and financial milestones that must be met to maintain the licensing rights to our current technology and intellectual property from the University of South Florida Research Foundation.
There are certain license fees and milestone payments required to be paid by us to the Licensor, pursuant to the terms of license agreements we have entered into with the Licensor. The license agreements for AL002 require us to pay royalty payments of 4% on net sales of products developed from the licensed technology for AL002 while the license agreements for AL001 require that we pay combined royalty payments of 4.5% on net sales of products developed from the licensed technology for AL001. We have already paid an initial license fee of $200,000 for AL002 and an initial license fee of $200,000 for AL001. As an additional licensing fee for the license of AL002, the Licensor received 3,601,809 shares of our common stock. As an additional licensing fee for the license of the AL001 technologies, the Licensor received 2,227,923 shares of our common stock. Minimum royalties for AL001 are $25,000 in 2023, $45,000 in 2024 and $70,000 in 2025 and every year thereafter, for the life of the agreement. Minimum royalties for AL002 are $20,000 in 2022, $40,000 in 2023 and $50,000 in 2024 and every year thereafter, for the life of the respective agreement. Additionally, we are required to pay milestone payments on the due dates to the Licensor for the license of the AL001 technologies and for the AL002 technology, as follows:
Original AL001 License:
| Payment | Due Date | Event | |||
| $ | 50,000 | * | Completed September 2019 | Pre-IND meeting | |
| $ | 65,000 | * | Completed June 2021 | IND application filing | |
| $ | 190,000 | * | Completed December 2021 | Upon first dosing of patient in a clinical trial | |
| $ | 500,000 | * | Completed March 2022 | Upon Completion of first clinical trial | |
| $ | 1,250,000 | 12 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 10,000,000 | 8 years from the effective date of the agreement | Upon FDA approval | ||
*Milestone met and completed
We have met the pre-IND meeting, IND application filing, and successfully completed the Phase I clinical trial milestones encompassing AL001. If we fail to meet a milestone payment by the specified date, the Licensor may terminate the respective license agreement. If the Licensor were to terminate either license agreement for whatever reason, it would materially and adversely affect our business, financial position and future prospects and you would likely lose the entirety of your investment in us.
AL002 License:
| Payment | Due Date | Event | |||
| $ | 50,000 | * | Completed January 2022 | Upon IND application filing | |
| $ | 50,000 | 12 months from IND application filing date | Upon first dosing of patient in first Phase I clinical trial | ||
| $ | 175,000 | 12 months from first patient dosed in Phase I | Upon completion of first Phase I clinical trial | ||
| $ | 500,000 | 24 months from completion of first Phase I clinical trial | Upon completion of first Phase II clinical trial | ||
| $ | 1,000,000 | 12 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 10,000,000 | 7 years from the effective date of the agreement | Upon FDA BLA approval | ||
*Milestone met and completed
| - 23 - |
On June 10, 2020, we obtained two additional royalty-bearing exclusive worldwide licenses from the Licensor to a therapy named AL001. One of the additional licenses is for the treatment of neurodegenerative diseases excluding Alzheimer’s and the other license is for the treatment of psychiatric diseases and disorders. There are certain license fees and milestone payments required to be paid pursuant to the terms of the June AL001 License Agreements. Under each of the June AL001 License Agreements, a royalty payment of 3% is required on net sales of products developed from the licensed technology. For the two additional AL001 licenses, in the aggregate, we paid initial license fees of $20,000. Additionally, under each of the June AL001 License Agreements, we are required to pay milestone payments on the due dates to the Licensor for the license of the technology, as follows:
Additional AL001 Licenses:
| Payment | Due Date | Event | |||
| $ | 50,000 | Upon IND application filing | IND application filing | ||
| $ | 150,000 | 12 months from IND filing date | Upon first dosing of patient in a clinical trial | ||
| $ | 400,000 | 12 months from first patient dosing | Upon Completion of first clinical trial | ||
| $ | 1,000,000 | 36 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 8,000,000 | 8 years from the effective date of the agreement | First commercial sale | ||
These June AL001 License Agreements have an indefinite term that continue until the later of the date no licensed patent under the applicable agreement remains a pending application or enforceable patent, the end date of any period of market exclusivity granted by a governmental regulatory body, or the date on which the licensee’s obligations to pay royalties expire under the applicable license agreement.
If we fail to comply with our obligations in the agreements under which we license intellectual property and other rights from third parties or otherwise experience disruptions to our business relationships with the Licensor, we could lose license rights that are important to our business.
We are a party to these license agreements with the Licensor and expect to enter into additional license agreements in the future. The existing license agreements impose, and we expect that future license agreements will impose, various diligence, milestone payment, royalty and other obligations on us. If we fail to comply with our obligations under these agreements, or we are subject to a bankruptcy, we may be required to make certain payments to the Licensor, we may lose the exclusivity of our license, or the Licensor may have the right to terminate the license, in which event we would not be able to develop or market products covered by the license. The Licensor or any future licensor may take any of these actions, including terminating a license agreement. Additionally, the milestone and other payments associated with these licenses will make it less profitable for us to develop our product candidates. If the Licensor were to terminate a license agreement for whatever reason, it would materially and adversely affect our business, financial position and future prospects and you would likely lose the entirety of your investment in us.
In some cases, patent prosecution of our licensed technology is controlled solely by the Licensor. If the Licensor fails to obtain and maintain patent or other protection for the proprietary intellectual property we license, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, and our competitors could market competing products using the intellectual property. Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues. Disputes may arise regarding intellectual property subject to a licensing agreement, including but not limited to:
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | the extent to which our technology and processes infringe on intellectual property of the Licensor that is not subject to the licensing agreement; |
| • | the sublicensing of patent and other rights; |
| • | our diligence obligations under each of the license agreements and what activities satisfy those diligence obligations; |
| • | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our collaborators; and |
| • | the priority of invention of patented technology. |
| - 24 - |
If disputes over intellectual property and other rights that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
We are substantially dependent on the success of our product candidates, which may not receive regulatory approval or be successfully commercialized.
In the future, we plan to submit AL001 and AL002 and, potentially, other product candidates for regulatory approval. Currently, however, neither AL001 nor AL002 has been submitted for regulatory approval, which would be required before we seek to initiate commercial distribution. To date, we have invested nearly all of our resources in establishing our company and the acquisition of the intellectual property of our product candidates, AL001 and AL002. Our near-term prospects, including our ability to finance our company and to enter into strategic collaborations and, ultimately, to generate revenue, are directly dependent upon the successful development, FDA approval and commercialization of AL001 or AL002.
The development and commercial success of our product will depend on a number of factors, including, without limitation, the following:
| • | our timely initiation and successful completion of preclinical studies and clinical trials for AL001 or AL002; |
| • | our demonstration to the satisfaction of the FDA and comparable regulatory bodies of the safety and efficacy of AL001 or AL002, as well as to obtain regulatory and marketing approval for AL001 or AL002 in the United States, Europe and elsewhere; |
| • | our continued compliance with all clinical and regulatory requirements applicable to AL001 and AL002; |
| • | our maintenance of an acceptable safety profile of AL001 and AL002 following regulatory approval; |
| • | competition with other treatments; |
| • | our creation, maintenance and protection of our intellectual property portfolio, including patents and trade secrets, and regulatory exclusivity for AL001 and AL002; |
| • | the effectiveness of our and our eventual partners’ marketing, sales and distribution strategy and operations; |
| • | the ability of our third-party manufacturers to manufacture supplies of our product and product candidates and to develop, validate and maintain commercially viable manufacturing processes; |
| • | our ability to launch commercial sales of AL001 or AL002 following regulatory approval, whether alone or in collaboration with others; and |
| • | the acceptance of AL001 and AL002 by physicians, health care payers, patients and the medical community. |
Many of these factors are beyond our control, and we cannot assure you that we will ever be able to generate sufficient revenue, or any revenue at all, from the sale of AL001 or AL002. Our failure in any of the above factors, or in successfully commercializing AL001 or AL002 on a timely basis, could have a material adverse effect on our business, results of operations and financial condition, and the value of your investment could substantially decline.
AL001 and AL002 may not achieve market acceptance, which would significantly limit our ability to generate revenue.
Even if we develop AL001 or AL002 and gain regulatory approvals for either or both candidates, unless physicians and patients accept our product candidates, we may not be able to sell them and generate significant revenues. We cannot assure you that AL001, AL002 or any other potential product candidates we may eventually develop will achieve market acceptance and revenue if and when they obtain the requisite regulatory approvals. Market acceptance of any product candidate depends on a number of factors, including but not limited to:
| • | the indication and warnings approved by regulatory authorities in the product label; |
| • | continued demonstration to the FDA of safety and efficacy in commercial use; |
| • | physicians’ willingness to prescribe the product; |
| - 25 - |
| • | reimbursement from third-party payers such as government health care systems and insurance companies; |
| • | the price of the product; |
| • | the nature of any post-approval risk management plans mandated by regulatory authorities; |
| • | competition; and |
| • | the effectiveness of marketing and distribution support. |
Any failure by AL001 or AL002 to achieve market acceptance or commercial success could have a material adverse effect on our business, results of operations and financial condition.
Problems in the manufacturing process, failure to comply with manufacturing regulations or unexpected increases in manufacturing costs could harm our business, results of operations and financial condition.
We are responsible for the manufacture and supply of AL001 and AL002, independently of each other. The manufacturing of AL001 and AL002 necessitates compliance with applicable regulatory requirements of the FDA and the European Union, as well as with international cGMP and other international regulatory requirements. As of the date of this Annual Report, we do not have our own manufacturing facilities. We have contracted with a third-party manufacturer for the clinical supply of AL001 using GMP manufacturing for our planned AL001 clinical trials and plan to contract with established third parties for the long-term commercial production of AL001 and AL002. The responsibility to obtain market authorization for AL001 and AL002 remains with us. As such, even if we could potentially have a claim against one or more third parties, we are legally liable for any noncompliance related to AL001 and AL002 and we expect to retain legal responsibility for any future product candidates as well.
Additionally, we may have limited control over the associated manufacturing costs and potential unexpected increases in those costs over time. If costs increase, we may choose to pass on such costs to our customers, which could reduce our ability to compete by increasing the prices of our products (which we expect to be priced at a significant premium over competing generic products). See “Risks Related to Our Business and Industry — We expect to face substantial competition, with other entities possibly discovering, developing or commercializing products before, or more successfully than, we do.” If we cannot pass on all such costs to our customers, then our profitability would be adversely affected.
If we are unable to manufacture, or contract to manufacture, AL001 and AL002 in accordance with regulatory specifications, or if there are disruptions in the manufacturing process due to damage, loss or failure to meet regulatory requirements (including passing inspections) of manufacturing facilities, we may not be able to meet the demand for our products or supply sufficient product for use in clinical trials, and this may harm our ability to commercialize AL001 and AL002 on a timely or cost-competitive basis, or preclude us from doing so at all, which could harm our business, results of operations and financial condition.
Before we or any future commercial partners can begin commercial manufacture of AL001 and AL002 or any other product candidate that we may develop in the future, we must obtain FDA regulatory approval for the product, which requires a successful FDA inspection of our manufacturing facilities (or those we contract with) and the development of quality systems, among other requirements. Even if we successfully pass an FDA Pre-Approval Inspection of any manufacturing facilities we may establish or contract with, our pharmaceutical facilities would be subject to unannounced inspection by the FDA and foreign regulatory authorities to ensure ongoing manufacturing compliance, even after product approval. Due to the complexity of the processes that we anticipate will eventually be used to manufacture AL001 and AL002, we may be unable to pass federal, state or international regulatory inspections in a cost-effective manner, whether initially or at any time thereafter. If we are unable to comply with manufacturing regulations, we may be subject to fines, unanticipated compliance expenses, recall or seizure of any approved products, or legal actions such as injunctions or criminal or civil prosecution. These possible sanctions could materially and adversely affect our business, results of operations and financial condition. See also “Risks Related to Development and Regulatory Approval of Our Product.” The regulatory approval process is uncertain, requires us to utilize significant financial, physical and human resources, and may prevent us or our future commercial partners from obtaining approvals for the commercialization of some or all of our product candidates.
| - 26 - |
Serious adverse events or other safety risks could require us to abandon development and preclude, delay or limit approval of AL001 or AL002, or limit the scope of any approved label or market acceptance.
If AL001, AL002 or any other product candidate that we may develop in the future, prior to or after any approval for commercial sale, causes serious or unexpected side effects, or become associated with other safety risks such as misuse, abuse or diversion, a number of potentially significant negative consequences could result, including, without limitation, that:
| • | regulatory authorities may interrupt, delay or halt clinical trials; |
| • | regulatory authorities may deny regulatory approval of AL001 or AL002; |
| • | regulatory authorities may require certain labeling statements, such as warnings or contraindications or limitations on the indications for use, or impose restrictions on distribution in the form of REMS in connection with approval, if any; |
| • | regulatory authorities may withdraw their approval, require more onerous labeling statements or impose a more restrictive REMS of any product that is approved; |
| • | we may be required to change the way the product is administered or conduct additional clinical trials; |
| • | any relationships that we may be able to form in the future with any commercial partners may suffer; |
| • | we could be sued and held liable for harm caused to patients; and |
| • | our reputation may suffer. |
We may voluntarily suspend or terminate our clinical trials if at any time we believe that they present an unacceptable risk to participants or if preliminary data demonstrate that either AL001 or AL002 is unlikely to receive regulatory approval or is unlikely to be successfully commercialized. In addition, regulatory agencies, an Ethics Committee or Institutional Review Board (an “IRB”), or data safety monitoring boards may at any time recommend the temporary or permanent discontinuation of our clinical trials or request that we cease using investigators in the clinical trials if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements, or that they present an unacceptable safety risk to participants. If we elect or are forced to suspend or terminate a clinical trial of AL001, AL002 or any other product candidate that we may in the future develop, the commercial prospects for that product will be harmed and our ability to generate product revenue from that product may be delayed or eliminated. Furthermore, any of these events could prevent us or our partners from achieving or maintaining market acceptance of the affected product and could substantially increase the costs of commercializing AL001 or AL002 and materially impair our ability to generate revenue from the commercialization of AL001 or AL002 either by us or by any future commercial partners with which we may develop a relationship, which and could have a material adverse effect on our reputation, business, results of operations and financial condition.
If we fail to obtain and sustain an adequate level of reimbursement for our products by third-party payers, sales and profitability will be adversely affected.
The course of medical treatment for human patients is, and will continue to be, expensive. We expect that most patients and their families will not be capable of paying for our potential products themselves.
Accordingly, it is unlikely that there will be a commercially viable market for AL001 or AL002, if approved, without reimbursement and coverage from third-party payers. Obtaining reimbursement approval and coverage from third-party payers is a time consuming and expensive process, and we cannot be certain that reimbursement will be approved and coverage obtained for our current product candidates or any other product candidate we may develop. Additionally, even if there is some form of reimbursement and coverage from third-party payers, if the level of third-party reimbursement is insufficient from the patient’s perspective or coverage is limited, our revenue and gross margins will be materially and adversely affected.
A current trend in the U.S. health care industry, as well as in other countries around the world, is toward cost containment. Large public and private payers, managed care organizations, group purchasing organizations and similar organizations are exerting increasing influence on decisions regarding the use of, and reimbursement levels for, particular treatments. Third-party payers, such as government programs, including Medicare in the United States, and private health care insurers, carefully review and have increasingly been challenging the coverage of, and prices charged for, medical products and services. Many third-party payers limit coverage of or reimbursement for newly-approved health care products. Reimbursement rates and coverage from private health insurance companies vary depending on the company, the insurance plan and other factors. Cost-control initiatives could decrease the price we or our partners establish for products, which could result in lower product revenue and profitability.
| - 27 - |
Reimbursement systems in international markets vary significantly by country and by region, and reimbursement approvals must be obtained on a country-by-country basis. Our eventual partners may elect to reduce the price of our products in order to increase the likelihood of obtaining reimbursement approvals. In many countries, products cannot be commercially launched until reimbursement is approved and the negotiation process in some countries can exceed 12 months. In addition, pricing and reimbursement decisions in certain countries can be affected by decisions taken in other countries, which can lead to mandatory price reductions and/or additional reimbursement restrictions across a number of other countries, which may adversely affect our sales and profitability. If countries set prices that are not sufficient to allow us or our partners to generate a profit, our partners may refuse to launch the product in such countries or withdraw the product from the market, which would adversely affect our sales and profitability and could materially and adversely affect our business, results of operations and financial condition.
Risks Related to Development and Regulatory Approval of Our Drug Candidates
The regulatory approval process is uncertain, requires us to utilize significant resources, and may prevent us or our future commercial partners from obtaining approvals for the commercialization of AL001 or AL002.
The research, testing, manufacturing, labeling, approval, sale, marketing and testing of AL001 and AL002 are and will be subject to extensive regulation by regulatory authorities in the United States, Europe and elsewhere, and regulatory requirements applicable to our product differ from country to country. Neither we nor any commercial partner is permitted to market any of our current or future product candidates in the United States until we receive approval from the FDA of either a NDA or BLA for AL001 and AL002, respectively. Obtaining approval of an NDA or a BLA can be an uncertain process that requires us to utilize significant resources. Furthermore, regulatory authorities possess broad discretion regarding processing time and usually request additional information and raise questions which have to be answered. There is considerable uncertainty regarding the times at which products may be approved and we have no control over the FDA review process. In addition, failure to comply with FDA and other applicable U.S. and foreign regulatory requirements may subject us to administrative or judicially imposed sanctions, including: warning letters, civil and criminal penalties, injunctions, withdrawal of approved products from the market, product seizure or detention, product recalls, total or partial suspension of production, and refusal to approve pending applications or supplements to approved applications.
Even if we fully comply with all applicable laws and regulations, the FDA may still determine that our clinical data are insufficient for final approval of an NDA or BLA. The process required by the FDA and most foreign regulatory authorities before human health care pharmaceuticals may be marketed generally involves nonclinical laboratory and, in some cases, animal tests; submission of an IND, which must become effective before clinical trials may begin; adequate and well-controlled human clinical trials to establish the safety and efficacy of the proposed drug for its intended use or uses; pre-approval inspection of manufacturing facilities and clinical trial sites; and FDA approval of an NDA or BLA, which must occur before a drug can be marketed or sold.
Regulatory approval of an NDA or BLA, or any supplement thereof, is not guaranteed, and the approval process requires us to utilize significant resources, could take several years, and is subject to the substantial discretion of the FDA. Despite the time and expense exerted, failure can occur at any stage, and we could encounter problems that cause us to abandon or have to repeat or perform additional studies. If our product or any of our future product candidates fails to demonstrate safety and efficacy in our studies, or for any other reason does not gain regulatory approval, our business and results of operations will be materially and adversely harmed.
In addition, separate regulatory approvals are required in order to market any product in many jurisdictions, including the United States, the United Kingdom, European Economic Area, which consists of the 27 Member States (known as the “EU Member States”) of the European Union plus Norway, Iceland and Liechtenstein, and many others. Approval procedures vary among countries and can involve additional studies and testing, and the time required to obtain approval may differ from that required to obtain FDA approval. Studies conducted in one country may not be accepted by regulatory authorities in other countries. Approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. The foreign regulatory approval process may include all of the risks associated with obtaining FDA approval. We may be unable to file for regulatory approvals or do so on a timely basis and, even if we are able to, we may not receive necessary approvals to commercialize our products in any market. Any of these results could have a material adverse effect on our business, results of operations and financial condition.
| - 28 - |
There is a high rate of failure for drug candidates proceeding through clinical trials.
Generally speaking, there is a high rate of failure for drug candidates proceeding through clinical trials. We may suffer significant setbacks in our clinical trials similar to the experience of a number of other companies in the pharmaceutical and biotechnology industries, even after receiving promising results in earlier trials. Further, even if we view the results of a clinical trial to be positive, the FDA or other regulatory authorities may disagree with our interpretation of the data. For instance, any such differing interpretation could cause the FDA to require additional trials. In the event that
| (i) | we obtain negative or inconclusive results from the AL001 or AL002 from a clinical trial, |
| (ii) | the FDA places a clinical hold on our clinical trials due to potential chemistry, manufacturing and controls issues or other hurdles, or |
| (iii) | the FDA does not approve our NDA for AL001 or our BLA for AL002, then: |
| • | we may not be able to generate sufficient revenue or obtain financing to continue our operations; |
| • | our ability to execute our current business plan will be materially impaired; |
| • | our reputation in the industry and in the investment community would likely be significantly damaged; and |
| • | the price of our common stock would likely decrease significantly. |
Any of these results could materially and adversely affect our business, results of operations or financial condition.
Nearly every attempt at drug approval for Alzheimer’s has failed.
Despite billions of dollars invested by the National Institute of Health and the biopharmaceutical industry in research programs to develop novel therapeutics for Alzheimer’s, the FDA has not approved any new drugs for Alzheimer’s since 2003, except, however, that in June 2021, aducanumab (Biogen, Inc) received approval from the FDA for the treatment of Alzheimer’s using the accelerated approval pathway. Since 2003, many new types and classes of drugs have been developed and tested in Alzheimer’s, including monoclonal antibodies, gamma secretase modulators and inhibitors, β-site amyloid precursor protein cleaving enzyme (BACE) inhibitors, receptor for advanced glycation end-products (RAGE) inhibitors, nicotinic partial agonists and allosteric modulators, serotonin subtype receptor (5HT6) antagonists, and others. Except for Biogen’s approval, referred to above, virtually all of these scientific programs have failed in clinical testing.
Clinical trials for AL001 or AL002 can be expensive, time consuming, uncertain and susceptible to change, delay or termination.
Clinical trials are expensive, time consuming and difficult to design and implement. The result of a clinical trial may be undesirable and can result in a clinical trial cancellation or the need for re-evaluation and supplementation. Even if the results of our clinical trials are favorable, the clinical trials for AL001 or AL002 are expected to continue for a few years and may even take significantly longer to complete. In addition, we, the FDA, an IRB, or other regulatory authority, whether in the United States, European Union or elsewhere, may suspend, delay or terminate our clinical trials at any time, for various reasons, including, without limitation:
| • | lack of effectiveness of AL001 or AL002 during clinical trials; |
| • | discovery of serious or unexpected toxicities or side effects experienced by trial participants or other safety issues; |
| • | slower than expected rates of subject recruitment and enrollment rates in clinical trials; |
| • | difficulty in retaining subjects who have initiated a clinical trial but may have withdrawn due to adverse side effects from the therapy, insufficient efficacy, fatigue with the clinical trial process or for any other reason; |
| • | delays or inability in manufacturing or obtaining sufficient quantities of materials for use in clinical trials due to manufacturing or regulatory constraints; |
| • | inadequacy of or changes in our manufacturing process or product formulation; |
| - 29 - |
| • | delays in obtaining regulatory authorization to commence a trial, including experiencing “clinical holds” or delays requiring suspension or termination of a trial by a regulatory agency, such as the FDA, before or after a trial is commenced; |
| • | changes in applicable regulatory policies and regulations; |
| • | delays or failure in reaching agreement on acceptable terms in clinical trial contracts or protocols with prospective clinical trial sites; |
| • | delay or failure to supply product for use in clinical trials which conforms to regulatory specification; |
| • | unfavorable results from ongoing preclinical studies and clinical trials; |
| • | failure of any contract research organizations (“CROs”) that we may partner with in the future, or other third-party contractors, to comply with all contractual requirements or to perform their services in a timely or acceptable manner; |
| • | failure by us, our employees, any CROs or their employees to comply with all applicable FDA or other regulatory requirements relating to the conduct of clinical trials; |
| • | scheduling conflicts with participating clinicians and clinical institutions; |
| • | failure to design appropriate clinical trial protocols; or |
| • | regulatory concerns with pharmaceutical products generally and the potential for abuse. |
The occurrence of any of the foregoing could have a material adverse effect on our business, results of operations and financial condition. See the risk factor “There is a high rate of failure for drug candidates proceeding through clinical trials” above.
If our products do not receive breakthrough therapy designation, it could potentially increase the FDA’s review time and adversely impact our development timeline. Even if the FDA grants breakthrough therapy designation, it does not guarantee faster product development or FDA review and does not necessarily increase the likelihood of the product candidates receiving approval from the FDA.
Breakthrough therapy designation is reserved for drug or biologic products that are intended to treat serious conditions and for which preliminary clinical evidence indicates that the candidate may demonstrate a substantial improvement on one or more clinically significant endpoints over currently available therapies. The benefits of receiving the designation include additional guidance from FDA throughout the development process, assistance with designing clinical trials, and coordination with FDA senior managers and experienced review staff. We plan to seek breakthrough therapy designation for both AL001 and AL002. However, we have not received breakthrough therapy designation or have qualified for expedited development, and no assurance can be given that we will. Even if we qualify for breakthrough therapy designation or expedited development, it may not actually lead to faster development or expedited regulatory review and approval or necessarily increase the likelihood that we will receive FDA approval.
Even if we believe that our products are strong candidates for breakthrough therapy designation, it is possible that the FDA may determine that our preliminary clinical evidence is insufficient to justify breakthrough therapy designation. Without this designation, we would not be able to benefit from the increased FDA guidance and assistance throughout the development process, and it is possible that our development timeline could be extended.
The breakthrough therapy designation, while advantageous for the development process for the reasons identified above, may nevertheless have little or no positive impact on our development process. There is no guarantee that, even with the FDA’s assistance through the breakthrough therapy designation, that the development process will be accelerated, the FDA will review or approve our submissions in a timely manner, or that our product candidates will ultimately receive approval from the FDA.
In summary, we cannot guarantee that our product candidates will receive breakthrough therapy designations and, even if they do, we cannot guarantee that such designations will have any bearing on the FDA’s review or approval of our product candidates.
| - 30 - |
Even if we receive regulatory approval for any of our future product candidates, we will be subject to ongoing FDA and other regulatory body obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates, if approved, will be subject to labeling and manufacturing requirements and could be subject to other restrictions. Failure to comply with these regulatory requirements or the occurrence of unanticipated problems with our products could result in significant penalties.
Any regulatory approvals that we or any of our collaborators receive for AL001, AL002 or any future product candidate may be subject to conditions of approval or limitations on the approved indicated uses for which the product may be marketed or may contain requirements for potentially costly surveillance to monitor the safety and efficacy of the product candidate. In addition, AL001, AL002 and any of our future product candidates, if approved by the FDA or other regulatory bodies, will be subject to extensive and ongoing regulatory requirements regarding the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion and recordkeeping. These requirements will include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMP, Good Laboratory Practice and Good Clinical Practice, the three types of audits related to the progressive stages needed to bring a pharmaceutical product to market, for any studies that we conduct post-approval. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with our third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on the marketing or manufacturing of the product, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| • | fines, warning letters or holds on target studies; |
| • | refusal by the FDA or other applicable regulatory body to approve pending applications or supplements to approved applications filed by us or our strategic collaborators, or suspension or revocation of product license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of products; and |
| • | injunctions or the imposition of civil or criminal penalties. |
The policies of the FDA and other regulatory bodies may change, and additional government regulations may be promulgated that could prevent, limit or delay regulatory approval of AL001 or AL002. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or elsewhere. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval that we may have obtained, and we may not achieve or sustain profitability, which would materially and adversely affect our business, results of operations and financial condition.
AL001 or AL002 and any of our future product candidates, if approved, may cause or contribute to adverse medical events that we are required to report to the FDA and regulatory authorities in other countries and, if we fail to do so, we could be subject to sanctions that would materially harm our business.
If we are successful in commercializing AL001, AL002 or any of our future product candidates, regulations promulgated by the FDA and by the regulatory authorities in other countries require that we report certain information about adverse medical events if those products may have caused or contributed to those adverse events. The timing of our obligation to report would be triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events we become aware of within the prescribed timeframe. We may also fail to appreciate that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of our products. If we fail to comply with our reporting obligations, the FDA and regulatory authorities in other countries could take action including criminal prosecution, the imposition of civil monetary penalties, seizure of our products, or delay in approval or clearance of future products, which could have a material adverse effect on our business, results of operations and financial condition.
Legislative or regulatory reforms with respect to products may make it more difficult and costly for us to obtain regulatory clearance or approval of AL001, AL002 or any of our future product candidates and to produce, market, and distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in the U.S. Congress and lawmaking bodies in other countries that could significantly change the statutory provisions governing the testing, regulatory clearance or approval, manufacture, and marketing of regulated products. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Similar changes in regulations can occur in other countries. Any new regulations or revisions or reinterpretations of existing regulations in the United States or in other countries may impose additional costs or lengthen review times of AL001, AL002 and any of our future product candidates. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
| • | requests for additional endpoints or studies; |
| - 31 - |
| • | changes to manufacturing methods; |
| • | recall, replacement, or discontinuance of certain products; and |
| • | additional record keeping. |
Each of these would likely entail substantial time and cost and could have a material adverse effect on our ability to obtain regulatory approval for our product candidates. In addition, delays in receipt of or failure to receive regulatory clearances or approvals for any future products could materially and adversely affect our business, results of operations and financial condition.
Our ability to market AL001, AL002 and any future product candidates in the United States, if approved, will be limited to use for the treatment of the indications for which they are approved, and if we want to expand the indications for which we may market AL001, AL002 and any future product candidates, we will need to obtain additional FDA approvals, which may not be granted.
We plan to seek full FDA approval in the United States for AL001 and AL002 to treat neurodegenerative diseases and psychiatric disorders, including Alzheimer’s. In addition, we have submitted a pre-IND meeting request with the FDA to explore AL001 for the treatment of bipolar disorder, MDD and PTSD. If AL001 or AL002 is approved, the FDA will restrict our ability to market or advertise it for the treatment of indications other than the one for which it is approved, which would limit its use. If we decide to attempt to develop, promote and commercialize new treatment indications and protocols for AL001, AL002 and potentially other product candidates in the future, we could not predict when, or if, we would ever receive the approvals required to do so. We would be required to conduct additional studies to support such applications for additional use, which would consume additional resources and may produce results that do not result in FDA approvals. If we do not obtain additional FDA approvals, our ability to expand our business in the United States would be adversely affected, which could materially and adversely affect our business, results of operations and financial condition.
The anticipated development of a REMS for AL001 or AL002 could cause delays in the approval process and would add additional layers of regulatory requirements that could impact our ability to commercialize AL001 and AL002 in the United States and reduce their market potential.
As a condition of approval of an NDA or a BLA, the FDA may require a REMS to ensure that the benefits of the drug outweigh the potential risks. REMS elements can include medication guides, communication plans for health care professionals, and elements to assure safe use (“ETASU”). ETASU’s can include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring, and the use of patient registries. Moreover, product approval may require substantial post-approval testing and surveillance to monitor the drug’s safety or efficacy. We may be required to adopt a REMS for AL001 or AL002 to ensure that the benefits outweigh the risks of abuse, misuse, diversion and other potential safety concerns. Even if the risk of abuse, misuse or diversion are not as high as for some other products, there can be no assurance that the FDA will approve a manageable REMS for AL001 or AL002, which could create material and significant limits on our ability to successfully commercialize AL001 and AL002 in the U.S. Delays in the REMS approval process could result in delays in the NDA or BLA approval process, respectively. In addition, as part of the REMS, the FDA could require significant restrictions, such as restrictions on the prescription, distribution and patient use of the product, which could significantly impact our ability to effectively commercialize AL001 or AL002, and dramatically reduce their market potential thereby adversely impacting our business, financial condition and results of operations. Even if initial REMS are not highly restrictive, if, after launch, AL001, AL002 and other drug candidates were to become subject to significant abuse/non-medical use or diversion from licit channels, this could lead to negative regulatory consequences, including a more restrictive REMS, which could materially and adversely affect our business, results of operations and financial condition.
If we are found in violation of “fraud and abuse” laws, we may be subject to criminal and civil penalties and/or be suspended or excluded from participation in government-run health care programs, which may adversely affect our business, financial condition and results of operations.
If we are successful in obtaining marketing approval for our products in the United States and elsewhere, we will be subject to various health care “fraud and abuse” laws, including anti-kickback laws, false claims laws and other laws intended to reduce fraud and abuse in government-run health care programs, which could materially and adversely affect us, particularly upon successful commercialization of our products in the United States. For example, the federal Anti-Kickback Statute makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer or pay any remuneration that is intended to induce the referral of business, including the purchase, order or prescription of a particular drug for which payment may be made under a U.S. health care program such as Medicare or Medicaid. Under U.S. federal government regulations, some arrangements, known as safe harbors, are deemed not to violate the Anti-Kickback Statute. Compliance with every element of a safe harbor regulation is required for the arrangement to be protected. However, arrangements that do not comply with a safe harbor are not per se illegal. Instead, they will be analyzed on a case-by-case basis. Although we intend to seek to structure our business arrangements in compliance with all applicable requirements, these laws are broadly written, and it is often difficult to determine precisely how the law will be applied in specific circumstances. Accordingly, it is possible that our practices may be challenged under the Anti-Kickback Statute and similar laws in other jurisdictions.
| - 32 - |
Further, false claims laws prohibit anyone from knowingly and willfully presenting or causing to be presented for payment to third-party payers, including government payers, reimbursement claims for drugs or services that are false or fraudulent, claims for items or services that were not provided as claimed, or claims for medically unnecessary items or services. Cases have been brought under false claims laws alleging that off-label promotion of pharmaceutical products or the payment of kickbacks by pharmaceutical providers has resulted in the submission of false claims to governmental health care programs. Under laws such as the Health Insurance Portability and Accountability Act of 1996 in the United States, we are prohibited from knowingly and willfully executing a scheme to defraud any health care benefit program, including private payers, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for health care benefits, items or services. Violations of fraud and abuse laws may be punishable by criminal and/or civil sanctions, including fines and/or exclusion or suspension from government-run health care programs such as Medicare and Medicaid and debarment from contracting with the U.S. and other governments. In addition, in the United States, individuals have the ability to bring actions on behalf of the government and potentially share in the recovery under the federal False Claims Act as well as under state false claims laws.
Many states in the United States have adopted fraud and abuse laws similar to their federal counterparts, including laws similar to the Anti-Kickback Statute, some of which apply to the referral of patients for health care services reimbursed by any source, not just governmental payers. In addition, California and some other states in the United States have passed laws that require pharmaceutical companies to comply with the April 2003 Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers and/or the Pharmaceutical Research and Manufacturers of America Code on Interactions with Health Care Professionals. In addition, several states impose other marketing restrictions or require pharmaceutical companies to make marketing or price disclosures to the state. There are ambiguities as to what is required to comply with these state requirements and if we fail to comply with an applicable state law requirement, we could be subject to penalties.
We have yet to receive definitive guidance on the application of fraud and abuse laws to our business. Law enforcement authorities are increasingly focused on enforcing these laws, and it is possible that some of our future practices may be challenged under these laws. While we believe we will be able to structure our business arrangements to comply with these laws, it is possible that the government could in the future allege violations of, or convict us of violating, these laws. If we are found in violation of one of these laws, we could be required to pay a penalty and could be suspended or excluded from participation in certain government-run health care programs, and our business, results of operations and financial condition may be materially and adversely affected.
Risks Related to Our Business and Industry
If we fail to attract and keep senior management and key scientific personnel, we may be unable to successfully develop AL001, AL002 or any future product candidates, conduct our in-licensing and development efforts or commercialize AL001, AL002 or any of our future product candidates.
Our future growth and success depend in part on our continued ability to attract, retain and motivate highly qualified management and scientific personnel. We are highly dependent upon our senior management, particularly Stephan Jackman, our Chief Executive Officer, Lien Escalona, our Chief Financial Officer, Kenneth S. Cragun, our Senior Vice President of Finance, Henry C.W. Nisser, our Executive Vice President and General Counsel, and David Katzoff, our Chief Operating Officer, as well as our consultants, Milton C. Ault, III, our Founder and Chairman Emeritus, Dr. Chuanhai Cao, the neuroscientist who developed AL002, and Dr. Roland (Doug) Shytle, one of the inventors of AL001. The loss of services of any of these individuals could delay or prevent the successful development of our current or future product pipeline, completion of our planned development efforts or the commercialization of AL001 or AL002. It is possible that current or former employees of ours could put forward claims for an alleged right to our patents and demand compensation therefor. If one or more of the key personnel were to leave us and engage in competing operations, our business, results of operations and financial condition could be materially and adversely affected.
| - 33 - |
We expect to face substantial competition, with other entities possibly discovering, developing or commercializing products before, or more successfully than, we do.
The development, FDA approval and commercialization of new therapy and vaccine products is highly competitive. We will face competition with respect to AL001, AL002 and any other product candidates that we may seek to develop or commercialize in the future, from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. In addition to existing therapeutic treatments for the indications we are targeting with AL001 and AL002, we also face potential competition from other drug candidates in development by other companies. Our potential competitors include large health care companies, such as Celgene Corporation, Merck & Co., Inc., Sanofi S.A., Eli Lilly and Company, Bayer AG, Novartis AG, Johnson and Johnson and Boehringer Ingelheim GmbH. We also know of several smaller early-stage companies that are developing products for use in our segment of the market. Some of the potential competitive compounds referred to above are being developed by large, well-financed and established pharmaceutical and biotechnology companies or have been partnered with such companies, which may give them development, regulatory and marketing advantages over our products.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, our ability to compete may be affected in many cases by insurers or other third-party payers seeking to encourage the use of generic products. If AL001 or AL002 achieves marketing approval, we expect that it will be priced at a significant premium over competing generic products.
Some of the companies against which we are competing or against which we may compete in the future have significantly greater financial, physical and human resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
If we are unable to compete successfully, we may be unable to grow and sustain our revenue, which could materially and adversely affect our business, results of operations and financial condition.
Changes in funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, or otherwise prevent our product candidates from being developed or commercialized in a timely manner, which could negatively impact our business.
We rely on the FDA to assist with the development our product candidates. The ability of the FDA to review and approve new drug products can be affected by a variety of factors outside of our control, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for our product candidates to be reviewed and/or potentially approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, including for 35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA employees and stop critical activities. If a prolonged government shutdown occurs, it could significantly impact the ability of the FDA to timely review and process our regulatory submissions, which could have a material adverse effect on our business. If the timing of FDA’s review and approval of new products is delayed, the estimated timing of our drug development program may be delayed which would materially increase costs of drug development and harm our operations or business.
Risks Related to Our Intellectual Property
We may be forced to litigate to enforce or defend our intellectual property rights, or the intellectual property rights of our licensors.
We may be forced to litigate to enforce or defend our intellectual property rights against infringement and unauthorized use by competitors. In so doing, we may place our intellectual property at risk of being invalidated, held unenforceable, or narrowed in scope. Further, an adverse result in any litigation or defense proceedings may place pending applications at risk of non-issuance. In addition, if any licensor fails to enforce or defend its intellectual property rights, this may adversely affect our ability to develop and commercialize AL001 or AL002 as well as our ability to prevent competitors from making, using, and selling competing products. Any such litigation could be very costly and could distract our management from focusing on operating our business. The existence or outcome of any such litigation could harm our business, results of operations and financial condition.
| - 34 - |
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential and proprietary information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock.
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
We rely on trade secrets to protect our proprietary know-how and technological advances, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights. Failure to obtain or maintain trade secret protection or failure to adequately protect our intellectual property could enable competitors to develop generic products or use our proprietary information to develop other products that compete with our products or cause additional, material adverse effects upon our business, results of operations and financial condition.
The transfer of technology and knowledge to contract manufacturers pursuant to the production of our products also creates a risk of uncontrolled distribution and copying of concepts, methods and processes relating to our products. Such uncontrolled distribution and copying could have a material adverse effect on the value of our products if used for the production of competing drugs or otherwise used commercially without our obtaining financial compensation.
We may become subject to third parties’ claims alleging infringement of patents and proprietary rights or seeking to invalidate our patents or proprietary rights, which would be costly, time-consuming and, if successfully asserted against us, delay or prevent the development and commercialization of AL001 or AL002.
There has been substantial litigation and other proceedings regarding patent and other intellectual property rights in the pharmaceutical industry, as well as patent challenge proceedings, including interference and administrative law proceedings before the USPTO and the European Patent Office (“EPO”), and oppositions and other comparable proceedings in other jurisdictions. Recently, under U.S. patent reform laws, new procedures including inter partes review and post grant review have been implemented. As stated below, the novel implementation of such laws presents uncertainty regarding the outcome of challenges to our patents in the future.
We cannot assure you that AL001, AL002 or any of our future product candidates will not infringe existing or future patents. We may be unaware of patents that have already issued that a third party might assert are infringed by AL001, AL002 or one of our future product candidates. Because patent applications can take many years to issue and may be confidential for 18 months or more after filing, there may be applications now pending of which we are unaware of and which may later result in issued patents that we may infringe by commercializing AL001, AL002 or any of our future product candidates. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. Moreover, we may face claims from non-practicing entities (commonly referred to as patent trolls), which have no relevant product revenue and against whom our own patent portfolio may thus have no deterrent effect.
We may be subject to third-party claims in the future against us or our collaborators that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages, including treble damages and attorney’s fees if we are found to be willfully infringing a third party’s patents. If a patent infringement suit were brought against us or our collaborators, we or our collaborators could be forced to stop or delay research, development, manufacturing or sales of AL001 or AL002. As a result of patent infringement claims, or in order to avoid potential claims, we or our collaborators may choose to seek, or be required to seek, a license from the third party and would most likely be required to pay license fees or royalties or both. These licenses may not be available on acceptable terms, or at all. Even if we or our collaborators were able to obtain a license, the rights may be nonexclusive, which would give our competitors access to the same intellectual property. Ultimately, we could be prevented from commercializing a product, or forced to redesign it, or to cease some aspect of our business operations if, as a result of actual or threatened patent infringement claims, we or our collaborators are unable to enter into licenses on acceptable terms. Even if we are successful in defending such claims, infringement and other intellectual property litigation can be expensive and time-consuming to litigate and divert management’s attention from our core business. Any of these events could harm our business significantly.
| - 35 - |
In addition to infringement claims against us, if third parties have prepared and filed patent applications in the U.S. that also claim technology to which we have rights, we may have to participate in interference proceedings in the USPTO to determine the priority of invention. Third parties may also attempt to initiate reexamination, post grant review or inter partes review of our patents in the USPTO. We may also become involved in similar opposition proceedings in the EPO or comparable offices in other jurisdictions regarding our intellectual property rights with respect to our products and technology. Any of these claims could have a material adverse effect on our business, results of operations and financial condition.
If our efforts to protect the proprietary nature of the intellectual property related to AL001, AL002 or any of our potential future product candidates are not adequate, we may not be able to compete effectively in our market.
We expect to rely upon a combination of patents, trade secret protection as well as confidentiality and license agreements to protect the intellectual property related to our product and our current product candidates and our development programs.
Composition-of-matter patents on an active pharmaceutical ingredient are generally considered to be the strongest form of intellectual property protection for pharmaceutical products, as such patents provide protection without regard to any particular method of use or manufacture. We cannot be certain that the claims in any patent application that we may submit covering composition-of-matter of AL001, AL002 and any potential future product candidates will be considered patentable by the USPTO and courts in the U.S., or by the patent offices and courts in foreign countries. Method-of-use patents protect the use of a product for the specified method. This type of patent does not prevent a competitor from making and marketing a product that is identical to our product for an indication that is outside the scope of the patented method.
The strength of patents involves complex legal and scientific questions and can be uncertain. The patent applications that we may in the future own or license may fail to result in issued patents in the United States or in other foreign countries. Even if the patents do successfully issue, third parties may challenge the validity, enforceability or scope thereof, which may result in such patents being narrowed, invalidated or held unenforceable. Furthermore, even if they are unchallenged, any of our future patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims. If the breadth or strength of protection provided by the patent applications we may own, license or pursue with respect to AL001, AL002 or any future product candidates is threatened, it could threaten our ability to commercialize AL001, AL002 or any future product candidates. Further, if we encounter delays in our development efforts, the period of time during which we could market AL001, AL002 or any future product candidates under patent protection would be reduced. Since patent applications in the U.S. and most other countries are confidential for a period of time after filing, we cannot be certain that we were the first to file any patent application related to AL001, AL002 or any future product candidates.
Even where laws provide protection, costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and the outcome of such litigation would be uncertain. Moreover, any actions we may bring to enforce our intellectual property against our competitors could provoke them to bring counterclaims against us, and some of our competitors have substantially greater intellectual property portfolios than we have.
We will also rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our product development processes that involve proprietary know-how, information or technology that is not covered by patents. Although we endeavor to execute confidentiality agreements with all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology, we cannot be certain that we have executed such agreements with all parties who may have helped to develop our intellectual property or had access to our proprietary information, nor that our agreements will not be breached. We cannot guarantee that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States or the European Union. As a result, we may encounter significant problems in protecting and defending our intellectual property not only in the United States and the European Union, but elsewhere as well. If we are unable to prevent material disclosure of the intellectual property related to our technologies to third parties, we will not be able to establish or maintain a competitive advantage in our market, which could materially and adversely affect our business, results of operations and financial condition and any disclosure to or misappropriation by third parties of our confidential proprietary information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding our competitive position in our market.
| - 36 - |
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect AL001 and AL002.
As is the case with other biopharmaceutical companies, our success will be heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involves both technological and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time-consuming and inherently uncertain. In addition, the U.S. has recently enacted and is currently implementing wide-ranging patent reform legislation. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in other situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in ways that would weaken our ability to obtain patents and to enforce patents that we might obtain in the future. Similarly, changes in EU patent law and elsewhere could negatively affect the value of our patents registered outside of the U.S.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with any of these requirements.
The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case, which could have a material adverse effect on our business, results of operations and financial condition.
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents on AL001, AL002 and any future product candidates throughout the world is prohibitively expensive. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection, but where enforcement is not as strong as that in the U.S. These products may compete with our products in jurisdictions where we do not have any issued or licensed patents and our patent claims or other intellectual property rights may not be effective or sufficient to prevent them from so competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biopharmaceuticals, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
Risks Relating to Legal Matters
We received a subpoena from the SEC in the investigation known as “In the Matter of DPW Holdings, Inc.,” the consequences of which are unknown.
In November 2019, we received a subpoena from the SEC that stated that the staff of the SEC is conducting an investigation known as “In the Matter of DPW Holdings, Inc.,” and that the subpoena was issued as part of an investigation as to whether BitNile Holdings, Inc., formerly known as DPW Holdings, Inc. (“BitNile”), and certain of its officers, directors, employees, partners, subsidiaries and/or affiliates, and/or other persons or entities, directly or indirectly, violated certain provisions of the Securities Act and the Exchange Act, in connection with the offer and sale of its securities. Although the order states that the SEC may have information relating to such alleged violations, the subpoena expressly provides that the inquiry is not to be construed as an indication by the SEC or its staff that any violations of the federal securities laws have occurred. We have produced documents in response to the subpoena. The SEC may in the future require us to produce additional documents, information or seek testimony from other members of our management team.
| - 37 - |
We are unaware of the scope or timing of the SEC’s investigation. As a result, we do not know how the SEC’s investigation is proceeding or when the investigation will be concluded. We also are unable to predict what action, if any, might be taken in the future by the SEC or its staff as a result of the matters that are the subject to its investigation or what impact, if any, the cost of continuing to respond to subpoenas might have on our financial position, results of operations, or cash flows. We have not established any provision for losses in respect of this matter. In addition, complying with any such future requests by the SEC for documents or testimony could distract the time and attention of our officers and directors or divert our resources away from ongoing business matters. This investigation could result in significant legal expenses, the diversion of management’s attention from our business, damage to our business and reputation, and could subject us to a wide range of remedies, including an enforcement action by the SEC. Two members of our current Board of Directors, Messrs. Horne and Nisser, are directors of BitNile. There can be no assurance that any final resolution of this and any similar matters will not have a material adverse effect on our business, financial condition or results of operations.
If product liability lawsuits are brought against us, we will incur substantial liabilities and may be required to limit the commercialization of AL001 or AL002.
We and our partners face potential product liability exposure related to the testing of AL001 or AL002 in clinical trials. We will face exposure to claims by an even greater number of persons if we begin to market and distribute our products commercially in the U.S. and elsewhere, including those relating to misuse of AL001 or AL002. Now, and in the future, an individual may bring a liability claim against us alleging that AL001 or AL002 caused an injury. While we intend to take what we believe to be appropriate precautions, we may be unable to avoid significant liability if any product liability lawsuit is brought against us. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities. Even if we successfully defend any such action, the costs associated with such defense could prove exorbitant. Regardless of merit or eventual outcome, liability claims may result in:
| • | decreased demand for AL001 or AL002 (if such product candidate had been approved and gone to market); |
| • | injury to our reputation; |
| • | withdrawal of clinical trial participants; |
| • | costs of related litigation; |
| • | substantial monetary awards to patients and others; |
| • | increased cost of liability insurance; |
| • | loss of revenue; and |
| • | our inability to successfully commercialize our products. |
Further, in the future there may be a need to expand the scope of our insurance coverage, which could result in significantly increased costs or the inability to obtain sufficient insurance coverage. Any of these occurrences could have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Our Affiliates’ Control and Relationships
Insiders currently have substantial influence over us, which could limit your ability to affect the outcome of key transactions, including a change of control.
In the aggregate, beneficial ownership of the shares of our common stock by our directors and executive officers and their respective affiliated parties represents approximately 48.2% of the outstanding shares of our common stock. As a result, these stockholders, if they act together, will be able to influence our management and affairs and all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions. This concentration of ownership may have the effect of delaying or preventing a change in control of our company and might affect the market price of our common stock.
Members of the Board of Directors and executive officers of our company and BitNile, contain some of the same individuals, which may present potential conflicts of interest.
Our company is controlled by Milton C. (Todd) Ault III, our Founder, Chairman Emeritus and consultant, directly and indirectly through his controlling equity interest in Ault & Company, Inc. the parent of Ault Life Sciences, Inc. and Ault Life Sciences Fund, LLC. Mr. Ault is also the Executive Chairman and single largest stockholder (through his control of Ault Alpha, LP) of BitNile, a publicly-traded diversified holding company focused primarily on the digital mining, investment, defense/aerospace, industrial and telecommunications industries. The Board of Directors and executive officers of our company and the board of directors and executive officers of BitNile contain some of the same individuals, all of whom devote a portion of their business and professional time and efforts to the respective businesses of our company as well as BitNile. William B. Horne, the Chairman of the Board of our company, is the Chief Executive Officer and a director of BitNile, Henry C.W. Nisser, our Executive Vice President, General Counsel and a director of our company, is the President, General Counsel and a director of BitNile, and Kenneth S. Cragun, our Senior Vice President of Finance is the Chief Financial Officer of BitNile. Additionally, Mr. Ault is the Chairman of Avalanche International, Corp. (“Avalanche”), a company currently engaged in developing advanced materials and processing technology for textile applications. Mr. Horne is a director of Avalanche and its Chief Financial Officer and Mr. Nisser is its Executive Vice President and General Counsel.
| - 38 - |
While we believe that our business and technologies are distinguishable from those of BitNile and that we do not compete in the markets in which BitNile compete, Mr. Ault and the other named individuals may have potential conflicts of interest with respect to, among other things, potential corporate opportunities, business combinations, joint ventures and/or other business opportunities that may become available to them, our company or BitNile. Moreover, while Mr. Ault and the other named individuals have agreed to devote a portion of their business and professional time and efforts to our company, potential conflicts of interest also include the amount of time and effort devoted by each of them to the affairs of BitNile. We may be materially adversely affected if Mr. Ault and/or the other named individuals choose to place the interests of BitNile before those of our company. Each of Mr. Ault and the other named individuals has agreed that, to the extent such opportunities arise, he will carefully consider a number of factors, including whether such opportunities were presented to him in his capacity as an officer or director of our company, whether such opportunities are within our company’s line of business or consistent with our strategic objectives and whether our company will be able to undertake or benefit from such opportunities. In addition, our Board of Directors has adopted a policy whereby any future transactions between us and any of our subsidiaries, affiliates, officers, directors, principal stockholders or any affiliates of the foregoing will be on terms no less favorable to our company than could reasonably be obtained in “arm’s length” transactions with independent third parties, and any such transactions will also be approved by a majority of our disinterested independent directors. The named individuals, other than Mr. Ault, owe fiduciary duties of care and loyalty to our company under Delaware law. However, the failure of our management to resolve any conflicts of interest in favor of our company could materially adversely affect our business, financial condition and results of operations.
Certain provisions of our certificate of incorporation allow concentration of voting power, which may, among other things, delay or frustrate the removal of incumbent directors or a takeover attempt, even if such events may be beneficial to our stockholders.
Provisions of our certificate of incorporation may delay or frustrate the removal of incumbent directors and may prevent or delay a merger, tender offer or proxy contest involving our company that is not approved by our Board of Directors, even if those events may be perceived to be in the best interests of our stockholders. Further, we may designate and issue separate classes of preferred stock that may entitle their holder(s) to exercise significant control over us. Consequently, anyone to whom or which these shares are or were issued could have sufficient voting power to significantly influence if not control the outcome of all corporate matters submitted to the vote of our common stockholders. Those matters could include the election of directors, changes in the size and composition of our Board, and mergers and other business combinations involving us. In addition, through any such person’s control of our Board and voting power, the affiliate may be able to control certain decisions, including decisions regarding the qualification and appointment of officers, dividend policy, access to capital (including borrowing from third-party lenders and the issuance of additional debt or equity securities), and the acquisition or disposition of assets by us. In addition, the concentration of voting power in the hands of an affiliate could have the effect of delaying or preventing a change in control of our company, even if the change in control could benefit our stockholders and may adversely affect the future market price of our common stock should a trading market therefor develop.
Risks Relating to Ownership of Our Common Stock
If we do not continue to satisfy the Nasdaq Capital Market continued listing requirements, our common stock could be delisted from the Nasdaq Capital Market.
The listing of our common stock on the Nasdaq Capital Market is contingent on our compliance with the Nasdaq Capital Market’s conditions for continued listing. We are currently not in compliance with Nasdaq listing requirements, specifically the minimum bid price requirement, and must regain compliance on or prior to December 19, 2022. If we are unable to regain such compliance, we will cease to be eligible to trade on Nasdaq.
If we were to fail to meet a Nasdaq Capital Market listing requirement, we may be subject to delisting by the Nasdaq Capital Market. In the event our common stock is no longer listed for trading on the Nasdaq Capital Market, our trading volume and share price may decrease and we may experience further difficulties in raising capital which could materially affect our operations and financial results. Further, delisting from the Nasdaq Capital Market could also have other negative effects, including potential loss of confidence by partners, lenders, suppliers and employees and could also trigger various defaults under our lending agreements and other outstanding agreements. Finally, delisting could make it harder for us to raise capital and sell securities. You may experience future dilution as a result of future equity offerings. In order to raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock.
| - 39 - |
We do not know whether an active market will be sustained; as a result, it may be difficult for you to sell your shares of our common stock.
If an active market for our common stock is not sustained, it may be difficult for you to sell your shares of common stock at an attractive price or at all. We cannot predict the prices at which our common stock will trade. It is possible that in one or more future periods our results of operations and progression of our product pipeline may not meet the expectations of public market analysts and investors and, as a result of these and other factors, the price of our common stock may fall.
The market price of our common stock is volatile, which could result in substantial losses for investors.
Our common stock is listed on the Nasdaq Capital Market. Since our initial public offering last year, our trading price has fluctuated widely, depending on many factors that may have little to do with our operations or business prospects. During the past 52-week period (through April 30, 2022), our stock closed at prices between $0.88 per share and $13.50 per share, as reported on Nasdaq.com.
Stock markets, in general, have experienced, and continue to experience, significant price and volume volatility, and the market price of our common stock may continue to be subject to similar market fluctuations unrelated to our operating performance or prospects. This increased volatility, coupled with depressed economic conditions, could continue to have a depressive effect on the market price of our common stock. The following factors, many of which are beyond our control, may influence our stock price:
| • | announcements of the failure to obtain regulatory approvals or receipt of a “complete response letter” from the FDA; |
| • | announcements of restricted label indications or patient populations, or changes or delays in regulatory review processes; |
| • | announcements of therapeutic innovations or new products by us or our competitors; |
| • | adverse actions taken by regulatory agencies with respect to our clinical trials, manufacturing supply chain or sales and marketing activities; |
| • | changes or developments in laws or regulations applicable to our product candidates; |
| • | any failure of our testing and clinical trials; |
| • | the impact of the ongoing COVID-19 pandemic on our business; |
| • | product liability claims, other litigation or public concern about the safety of our product candidates or future products; |
| • | any adverse changes to our relationship with licensors, manufacturers or suppliers; |
| • | the loss of any of our key scientific or management personnel; |
| • | any major changes to our Board of Directors or management; |
| • | the failure to obtain new commercial partners; |
| • | announcements concerning our competitors or the pharmaceutical industry in general; |
| • | the failure to achieve expected product sales and profitability; |
| • | the failure to obtain reimbursements for our product candidates as part of any healthcare insurance plan, or reductions in such reimbursements; |
| • | actual or anticipated fluctuations in our cash position or operating results; |
| • | manufacturing, supply or distribution shortages related to our current or future product candidates for our development programs and commercialization; |
| • | changes in financial estimates or recommendations by securities analysts; |
| - 40 - |
| • | the termination of any of our existing license agreements; |
| • | announcements relating to future licensing or development agreements; |
| • | potential acquisitions; |
| • | the trading volume of shares on The Nasdaq Capital Market; |
| • | sales of our shares by us, our executive officers or directors or our shareholders; |
| • | fluctuations in the U.S. equity markets; |
| • | changes in accounting principles; |
| • | market conditions in the healthcare sector; and |
| • | general economic conditions in the United States and elsewhere. |
In recent years, each of the stock market in general, and the market for pharmaceutical and biotechnology companies in particular, has experienced significant price and volume fluctuations that have often been unrelated or disproportionate to changes in the operating performance of the companies whose stock is experiencing those price and volume fluctuations. Broad market and industry factors may seriously affect the market price of our common stock, regardless of our actual operating performance. Following periods of such volatility in the market price of a company’s securities, securities class action litigation has often been brought against that company. Because of the potential volatility of our stock price, we may become the target of securities litigation in the future. Securities litigation could result in substantial costs and divert management’s attention and resources from our business.
If there are substantial sales of shares of our common stock, the price of our common stock could decline.
The price of our common stock could decline if there are substantial sales of our common stock, particularly sales by our directors, executive officers and significant stockholders, or if there is a large number of shares of our common stock available for sale and the market perceives that sales will occur. As of July 19, 2022, we had 95,481,790 shares of our common stock outstanding. Shares held by directors, executive officers and other affiliates will be subject to volume limitations under Rule 144 under the Securities Act and various vesting agreements. We have registered shares of common stock that we have issued and may issue under our employee equity incentive plans, which shares may be sold freely in the public market upon issuance. Sales of our common stock by current stockholders may make it more difficult for us to sell equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate, and make it more difficult for other stockholders to sell shares of our common stock.
The market price of the shares of our common stock could decline as a result of the sale of a substantial number of our shares of common stock in the public market or the perception in the market that the holders of a large number of shares intend to sell their shares. We are unable to predict the effect that sales may have on the prevailing market price of our common stock.
The concentration of our stock ownership will limit your ability to influence corporate matters, including the ability to influence the outcome of director elections and other matters requiring stockholder approval.
Our executive officers, directors and the holders of more than 5% of our outstanding common stock, in the aggregate, beneficially own a significant percentage of our common stock. As a result, these stockholders, acting together, will have significant influence over all matters that require approval by our stockholders, including the election of directors and approval of significant corporate transactions. Corporate actions might be taken even if other stockholders oppose them. This concentration of ownership might also have the effect of delaying or preventing a change of control of our company that other stockholders may view as beneficial.
Our bylaws provide that the Court of Chancery of the State of Delaware and the federal district courts of the United States are the exclusive forums for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our bylaws provides that the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising under the Delaware General Corporation Law, our certificate of incorporation or our bylaws; any action to interpret, apply, enforce or determine the validity of our certificate of incorporation or our bylaws; and any action asserting a claim against us that is governed by the internal affairs doctrine. This provision would not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the U.S. federal courts have exclusive jurisdiction.
| - 41 - |
Our bylaws further provide that the federal district courts of the United States will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. The enforceability of similar exclusive federal forum provisions in other companies’ organizational documents has been challenged in legal proceedings, and while the Delaware Supreme Court has ruled that this type of exclusive federal forum provision is facially valid under Delaware law, there is uncertainty as to whether other courts would enforce such provisions and that investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder.
These exclusive forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits against us and our directors, officers and other employees. Alternatively, if a court were to find either exclusive forum provision in our bylaws to be inapplicable or unenforceable in an action, we may incur further significant additional costs associated with resolving such action in other jurisdictions, all of which could have a material adverse effect on our business, financial condition, and results of operations.
Because we do not intend to pay dividends on our common stock, you must rely on stock appreciation for any return on your investment.
We presently intend to retain any future earnings and do not expect to pay any dividends in the foreseeable future. As a result, you must rely on stock appreciation and a liquid trading market for any return on your investment. If an active and liquid trading market does not develop, you may be unable to sell your shares of common stock at or above the initial public offering price or at the time you would like to sell.
We have identified a material weakness in our internal control over financial reporting. If our remediation of this material weakness is not effective, or if we experience additional material weaknesses in the future or otherwise fail to maintain an effective system of internal controls in the future, we may not be able to accurately or timely report our financial condition or results of operations, which may adversely affect investor confidence in us and, as a result, the value of our common stock.
We have limited accounting personnel to adequately execute our accounting processes and other supervisory resources with which to address our internal control over financial reporting. In connection with the audit of our financial statements for the year ended April 30, 2022, we identified material weaknesses in our internal control over financial reporting. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of our financial statements will not be prevented or detected on a timely basis. The material weaknesses related to a lack of sufficient number of qualified personnel within our accounting function to adequately segregate duties, to perform sufficient reviews and approval of manual journal entries posted to the general ledger and to consistently execute review procedures over general ledger account reconciliations, financial statement preparation and accounting for non-routine transactions and, we have not designed and implemented effective Information Technology General Controls (“ITGC”) related to access controls to payment and financial accounting systems.
We are implementing measures designed to improve our internal control over financial reporting to remediate this material weakness, including the following:
| • | We are formalizing our internal control documentation and strengthening supervisory reviews by our management; |
| • | We are in the process of adding additional accounting personnel and segregating duties amongst accounting personnel; and |
| • | We are in the process of strengthening ITGC access controls related to our payment and financial accounting systems. |
We cannot assure you that the measures we have taken to date, and are continuing to implement, will be sufficient to remediate the material weakness we have identified or avoid potential future material weaknesses. If the steps we take do not correct the material weakness in a timely manner, we will be unable to conclude that we maintain effective internal control over financial reporting. Accordingly, there could continue to be a reasonable possibility that a material misstatement of our financial statements would not be prevented or detected on a timely basis.
As a public company, we are required to maintain internal control over financial reporting and to report any material weaknesses in such internal controls. We must perform system and process evaluation and testing of our internal controls over financial reporting to allow management to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. The Sarbanes-Oxley Act also requires that our management report on internal control over financial reporting be attested to by our independent registered public accounting firm, to the extent we are no longer an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012 (JOBS Act). We do not expect our independent registered public accounting firm to attest to our management report on internal control over financial reporting for so long as we are an emerging growth company.
| - 42 - |
We are in the process of enhancing our internal control over financial reporting required to comply with this obligation, which process will be time consuming, costly and complicated. If we identify any additional material weaknesses in our internal control over financial reporting, if we are unable to comply with the requirements of Section 404 in a timely manner, if we are unable to assert that our internal control over financial reporting is effective, or when required in the future, if our independent registered public accounting firm is unable to express an opinion as to the effectiveness of our internal control over financial reporting, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our common stock could be adversely affected, and we could become subject to investigations by the Nasdaq Stock Market, the SEC, or other regulatory authorities, which could require additional financial and management resources.
General Risk Factors
We must effectively manage the growth of our operations, or our company will suffer.
Our initiation of operations has resulted in significantly higher operating expenses. Expansion of our operations, to include the development of AL001 and AL002, may also cause a significant demand on our management, finances and other resources. Our ability to manage the anticipated future growth, should it occur, will depend upon a significant expansion of our accounting and other internal management systems and the implementation and subsequent improvement of a variety of systems, procedures and controls. In addition, we intend to expand our scientific advisory board. There can be no assurance that significant problems in these areas will not occur. Any failure to expand these areas and implement and improve AL001 or AL002 or our procedures and controls in an efficient manner at a pace consistent with our business could have a material adverse effect on our business, financial condition and results of operations. There can be no assurance that our attempts to expand our marketing, sales, manufacturing and customer support efforts will be successful or will result in additional sales or profitability in any future period.
We may not be successful in our efforts to expand our pipeline of product candidates.
One element of our strategy is to expand our pipeline of pharmaceuticals based on our technology and advance these product candidates through clinical development for the treatment of a variety of indications. Although our research and development efforts to date have resulted in a number of development programs based on our technology, we may not ultimately be able to develop product candidates that are safe and effective. Even if we are successful in continuing to expand our pipeline, the potential product candidates that we identify may not be suitable for clinical development, including as a result of being shown to have harmful side effects or other characteristics that indicate that they are unlikely to receive marketing approval and achieve market acceptance. In addition, if we attempt to apply our technology to develop product candidates for indications outside of Alzheimer’s, we will need to evaluate the preclinical data and determine if additional data are needed to support the new indications. If we do not successfully develop and commercialize product candidates based upon our technological approach, we will not be able to obtain product revenue in future periods, which would make it unlikely that we would ever achieve profitability.
We may experience product recalls or inventory losses caused by unforeseen events, cold chain interruption and testing difficulties.
AL001 and AL002, individually, will be manufactured and distributed, if ever, using technically complex processes requiring specialized facilities, highly specific raw materials and other production constraints. The complexity of these processes, as well as the strict company and government standards for the manufacture of our products, will subject us to production risks. While product batches released for use in clinical trials or for commercialization undergo sample testing, some defects may only be identified following product release. In addition, process deviations or unanticipated effects of approved process changes may result in these intermediate products not complying with stability requirements or specifications. Most of our products must be stored and transported at temperatures within a certain range, which is known as “strict cold chain” storage and transportation. If these environmental conditions deviate from the norm, our products’ remaining shelf lives could be impaired or their quality could become adversely affected, making them no longer suitable for use. The occurrence or suspected occurrence of production and distribution difficulties can lead to lost inventories, and in some cases product recalls, with consequential reputational damage and the risk of product liability. The investigation and remediation of any identified problems can cause production delays, substantial expense, lost sales and delays of new product launches, any of which could have a material adverse effect on our business, results of operations and financial condition.
| - 43 - |
We may have trouble hiring additional qualified personnel.
As we expand our development and commercial activities, we will need to hire additional personnel and could experience difficulties attracting and retaining qualified employees. Competition for qualified personnel in the biopharmaceutical field is intense due to the limited number of individuals who possess the skills and experience required by that industry. We may not be able to attract and retain quality personnel on favorable terms, or at all. In addition, to the extent we hire personnel from competitors, we may be subject to allegations that such personnel have been improperly solicited or that they have divulged proprietary or other confidential information, or that their former employers own their research output. Any of these difficulties could have a material adverse effect on our business, results of operations and financial condition.
Failure of our information technology systems could significantly disrupt the operation of our business.
Our ability to execute our business plan and to comply with regulatory requirements with respect to data control and data integrity depends, in part, on the continued and uninterrupted performance of our information technology systems, or IT systems. These systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our IT systems, there are no assurances that electronic break-ins, computer viruses and similar disruptive problems, and/or sustained or repeated system failures or problems arising during the upgrade of any of our IT systems that interrupt our ability to generate and maintain data will not occur. The occurrence of any of the foregoing with respect to our IT systems could have a material adverse effect on our business, results of operations or financial condition.
We are subject to various claims and legal actions arising in the ordinary course of our business.
We are subject to various claims and legal actions arising in the ordinary course of our business. Any such litigation could be very costly and could distract our management from focusing on operating our business. The existence of any such litigation could harm our business, results of operations and financial condition. Results of actual and potential litigation are inherently uncertain. An unfavorable result in a legal proceeding could adversely affect our reputation, financial condition and operating results.
We will be subject to the U.S. Foreign Corrupt Practices Act and other anti-corruption laws, as well as export control laws, customs laws, sanctions laws and other laws governing our anticipated operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures, and legal expenses, which could adversely affect our business, results of operations and financial condition.
Our operations, if initiated, will be subject to certain anti-corruption laws, including the U.S. Foreign Corrupt Practices Act (“FCPA”), and other anti-corruption laws that apply in countries where we do business. The FCPA and other anti-corruption laws generally prohibit us and our employees and intermediaries from bribing, being bribed or making other prohibited payments to government officials or other persons to obtain or retain business or gain some other business advantage. We and any future commercial partners may operate in a number of jurisdictions that pose a high risk of potential FCPA violations and we may participate in collaborations and relationships with third parties whose actions could potentially subject us to liability under the FCPA or local anti-corruption laws. In addition, we cannot predict the nature, scope or effect of future regulatory requirements to which our international operations might be subject or the manner in which existing laws might be administered or interpreted.
We also anticipate becoming subject to other laws and regulations governing our international operations, including regulations administered in the U.S. and in the EU, including applicable export control regulations, economic sanctions on countries and persons, customs requirements and currency exchange regulations (collectively, “Trade Control Laws”).
There can be no assurance that we will be completely effective in ensuring our compliance with all applicable anticorruption laws, including the FCPA or other legal requirements, such as Trade Control Laws. Any investigation of potential violations of the FCPA, other anti-corruption laws or Trade Control Laws by the United States, the European Union or other authorities could have an adverse impact on our reputation, our business, results of operations and financial condition. Furthermore, should we be found not to be in compliance with the FCPA, other anti-corruption laws or Trade Control Laws, we may be subject to criminal and civil penalties, disgorgement and other sanctions and remedial measures, as well as the accompanying legal expenses, any of which could have a material adverse effect on our reputation and liquidity, as well as on our business, results of operations and financial condition.
| - 44 - |
Certain provisions of our certificate of incorporation, bylaws and Delaware law make it more difficult for a third party to acquire us and make a takeover more difficult to complete, even if such a transaction were in the stockholders’ interest.
Our certificate of incorporation, bylaws and certain provisions of Delaware law could have the effect of making it more difficult or more expensive for a third party to acquire, or discouraging a third party from attempting to acquire, control of our company, even when these attempts may be in the best interests of our stockholders. For example, we are governed by Section 203 of the Delaware General Corporation Law. In general, Section 203 prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes mergers, asset sales or other transactions resulting in a financial benefit to the stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years did own, 15% or more of the corporation’s outstanding voting stock. These provisions may have the effect of delaying, deferring or preventing a change in control of our company.
Failure to build our finance infrastructure and improve our accounting systems and controls could impair our ability to comply with the financial reporting and internal controls requirements for publicly traded companies.
As a public company, we operate in an increasingly demanding regulatory environment, which requires us to comply with the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, the regulations of The Nasdaq Capital Market, the rules and regulations of the SEC, expanded disclosure requirements, accelerated reporting requirements and more complex accounting rules. Company responsibilities required by the Sarbanes-Oxley Act include establishing corporate oversight and adequate internal control over financial reporting and disclosure controls and procedures. Effective internal controls are necessary for us to produce reliable financial reports and are important to help prevent financial fraud. We must perform system and process evaluation and testing of our internal controls over financial reporting to allow management to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act.
We anticipate that the process of building our accounting and financial functions and infrastructure will require significant additional professional fees, internal costs and management efforts. We expect that we will need to implement a new internal system to combine and streamline the management of our financial, accounting, human resources and other functions. However, such a system would likely require us to complete many processes and procedures for the effective use of the system or to run our business using the system, which may result in substantial costs. Any disruptions or difficulties in implementing or using such a system could adversely affect our controls and harm our business. Moreover, such disruption or difficulties could result in unanticipated costs and diversion of management attention. In addition, we may discover weaknesses in our system of internal financial and accounting controls and procedures that could result in a material misstatement of our financial statements. Our internal control over financial reporting will not prevent or detect all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud will be detected.
If we are not able to comply with the requirements of Section 404 of the Sarbanes-Oxley Act in a timely manner, or if we are unable to maintain proper and effective internal controls, we may not be able to produce timely and accurate financial statements. If we cannot provide reliable financial reports or prevent fraud, our business and results of operations could be harmed, investors could lose confidence in our reported financial information and we could be subject to sanctions or investigations by The Nasdaq Capital Market, the SEC or other regulatory authorities.
If securities analysts do not publish research or reports about our business or if they publish negative evaluations of our stock, the price of our common stock could decline.
The trading market for our common stock will rely in part on the research and reports that industry or financial analysts publish about us or our business. We do not currently have and may never obtain research coverage by industry or financial analysts. If no or few analysts commence coverage of us, the trading price of our common stock could decrease. Even if we do obtain analyst coverage, if one or more of the analysts covering our business downgrade their evaluations of our stock, the price of our common stock could decline. If one or more of these analysts cease to cover our stock, we could lose visibility in the market for our common stock, which in turn could cause our stock price to decline.
| - 45 - |
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. For so long as we remain an emerging growth company, we are permitted and plan to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of SOX Section 404, not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. As a result, the information we provide stockholders will be different than the information that is available with respect to other public companies. In this Annual Report, we have not included all of the executive compensation-related information that would be required if we were not an emerging growth company. We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile.
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and particularly after we are no longer an emerging growth company (or, to a lesser extent, a smaller reporting company), we will incur significant legal, accounting, and other expenses that we did not incur as a private company. Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The Nasdaq Capital Market, and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. We expect that we will need to hire additional accounting, finance, and other personnel in connection with our becoming, and our efforts to comply with the requirements of being, a public company, and our management and other personnel will need to devote a substantial amount of time towards maintaining compliance with these requirements. These requirements will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, we expect that the rules and regulations applicable to us as a public company may make it more difficult and more expensive for us to obtain director and officer liability insurance, which could make it more difficult for us to attract and retain qualified members of our Board of Directors. We are currently evaluating these rules and regulations and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Our charter provides for limitations of director liability and indemnification of directors and officers and employees.
Our certificate of incorporation limits the liability of directors to the maximum extent permitted by Delaware law. Delaware law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
| • | breach of their duty of loyalty to us or our stockholders; |
| • | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| • | transaction from which the directors derived an improper personal benefit. |
These limitations of liability do not apply to liabilities arising under the federal or state securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission.
Our bylaws provide that we will indemnify our directors, officers and employees to the fullest extent permitted by law. Our bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding. We believe that these provisions are necessary to attract and retain qualified persons as directors and officers.
The limitation of liability in our certificate of incorporation and bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might provide a benefit to us and our stockholders. Our results of operations and financial condition may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
| - 46 - |
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because biopharmaceutical companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS |
None.
| ITEM 2. | PROPERTIES |
Our executive office is currently located at 3500 Lenox Rd NE, Suite 1500, Atlanta, GA 30326, where we utilize shared labs and extensive research resources. Our accounting and finance office is located in Orange County, California utilizing approximately 200 square feet of shared office space within the offices of BitNile, a related party. Our legal office is located in New York, New York utilizing shared office space within the offices of BitNile. We currently do not pay rent for our Orange County, California or New York, New York office spaces. We believe our present space is adequate for our current operations.
| ITEM 3. | LEGAL PROCEEDINGS |
We are subject to various claims and legal actions arising in the ordinary course of our business. Any such litigation could be very costly and could distract our management from focusing on operating our business. The existence of any such litigation could harm our business, results of operations and financial condition. Results of actual and potential litigation are inherently uncertain. An unfavorable result in a legal proceeding could adversely affect our reputation, financial condition and operating results. There are no legal proceedings or arbitration proceedings currently pending against our company.
| ITEM 4. | MINE SAFETY DISCLOSURES |
Not applicable.
| - 47 - |
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock began trading on The NASDAQ Capital Market under the symbol “ALZN” on June 15, 2021. Prior to that date, there was no public trading market for our common stock.
Holders of Record
As of July 19, 2022, there were approximately 133 stockholders of record of our common stock. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust or by other entities.
Dividend Policy
We have never declared or paid any cash dividends on our capital stock, and we do not currently intend to pay any cash dividends on our capital stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to support operations and to finance the growth and development of our business. Any future determination to pay dividends will be made at the discretion of our Board of Directors subject to applicable laws and will depend upon, among other factors, our results of operations, financial condition, contractual restrictions and capital requirements. Our future ability to pay cash dividends on our capital stock may be limited by any future debt instruments or preferred securities.
Equity Compensation Information
The information required by this item regarding equity compensation plans is incorporated by reference to the information set forth in Item 12 of this Annual Report on Form 10-K.
Recent Sales of Unregistered Securities
On April 26, 2022, we issued and sold 2,666,667 shares of our common stock to Digital Power Lending, LLC (“DPL”) for $4 million, or $1.50 per share, and issued to DPL warrants to acquire 1,333,333 shares of its common stock with an exercise price of $3.00 per share pursuant to the March 12, 2021 securities purchase agreement.
Use of Proceeds
On June 15, 2021, we issued and sold 2,875,000 shares of our common stock in the initial public offering (“IPO”) at a public offering price of $5.00 per share, resulting in net proceeds of $12.9 million after deducting underwriting discounts and commissions and offering expenses paid by us.
There has been no material change in our planned use of the net proceeds from our IPO as described in our final prospectus filed pursuant to Rule 424(b)(4) under the Securities Act with the SEC. As of April 30, 2022, we have used $6.8 million of the net proceeds from the IPO.
| ITEM 6. | [RESERVED] |
| - 48 - |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
You should read the following discussion and analysis of our financial condition and results of our operations together with our financial statements and the notes thereto appearing elsewhere in this Annual Report. This discussion contains forward-looking statements reflecting our current expectations, whose actual outcomes involve risks and uncertainties. Actual results and the timing of events may differ materially from those stated in or implied by these forward-looking statements due to a number of factors, including those discussed in the sections entitled “Risk Factors” and “Special Note Regarding Forward-Looking Statements,” and elsewhere in this Annual Report.
Overview
We were incorporated on February 26, 2016 as Alzamend Neuro, Inc. under the laws of the State of Delaware. We were formed to acquire and commercialize patented intellectual property and know-how to prevent, treat and cure the crippling and deadly Alzheimer’s. Existing Alzheimer’s treatments only temporarily relieve symptoms but do not slow or halt the underlying worsening of the disease. We have developed a novel approach in an attempt to combat Alzheimer’s through immunotherapy.
Critical Accounting Policies and Estimates
Research and Development Expenses. Research and development costs are expensed as incurred. Research and development costs consist of scientific consulting fees and lab supplies, as well as fees paid to other entities that conduct certain research and development activities on behalf of our company.
We have acquired and may continue to acquire the rights to develop and commercialize new product candidates from third parties. The upfront payments to acquire license, product or rights, as well as any future milestone payments, are immediately recognized as research and development expense provided that there is no alternative future use of the rights in other research and development projects.
Stock-Based Compensation. We maintain a stock-based compensation plan as a long-term incentive for employees, non-employee directors and consultants. The plan allows for the issuance of incentive stock options, non-qualified stock options, restricted stock units, and other forms of equity awards.
We recognize stock-based compensation expense for stock options on a straight-line basis over the requisite service period and account for forfeitures as they occur. Our stock-based compensation costs are based upon the grant date fair value of options estimated using the Black-Scholes option pricing model. To the extent any stock option grants are made subject to the achievement of a performance-based milestone, management evaluates when the achievement of any such performance-based milestone is probable based on the relative satisfaction of the performance conditions as of the reporting date.
The Black-Scholes option pricing model utilizes inputs which are highly subjective assumptions and generally require significant judgment. These assumptions include:
| · | Fair Value of Common Stock. See the subsection titled “– Common Stock Valuations” below; |
| · | Risk-Free Interest Rate. The risk-free interest rate is based on the U.S. Treasury zero coupon issues in effect at the time of grant for periods corresponding with the expected term of the option; |
| · | Expected Volatility. Because we do not have an extensive trading history for our common stock, the expected volatility was estimated based on the average volatility for comparable publicly traded life sciences companies over a period equal to the expected term of the stock option grants. The comparable companies were chosen based on the similar size, stage in life cycle or area of specialty. We will continue to apply this process until a sufficient amount of historical information regarding the volatility of our own stock price becomes available; |
| · | Expected Term. The expected term represents the period that the stock-based awards are expected to be outstanding and is determined using the simplified method (based on the mid-point between the vesting date and the end of the contractual term), as we do not have sufficient historical data to use any other method to estimate expected term; and |
| · | Expected Dividend Yield. We have never paid dividends on our common stock and have no plans to pay dividends on our common stock. Therefore, we used an expected dividend yield of zero. |
| - 49 - |
Certain of such assumptions involve inherent uncertainties and the application of significant judgment. As a result, if factors or expected outcomes change and we use significantly different assumptions or estimates, our stock-based compensation could be materially different.
Common Stock Valuations. Prior to our IPO in June 2021, there was no public market for our common stock, and, as a result, the fair value of the shares of common stock underlying our share-based awards was estimated on each grant date by our Board of Directors. To determine the fair value of our common stock underlying option grants, our Board of Directors considered, among other things, input from management, and our Board of Directors’ assessment of additional objective and subjective factors that it believed were relevant, and factors that may have changed from the date of the most recent valuation through the date of the grant. These factors included, but were not limited to:
| · | our results of operations and financial position, including our levels of available capital resources; |
| · | our stage of development and material risks related to our business; |
| · | progress of our research and development activities; |
| · | our business conditions and projections; |
| · | the valuation of publicly traded companies in the life sciences and biotechnology sectors, as well as recently completed mergers and acquisitions of peer companies; |
| · | the lack of marketability of our common stock as a private company; |
| · | the prices at which we sold shares of our common stock to outside investors in arms-length transactions; |
| · | the likelihood of achieving a liquidity event for our security holders, such as an initial public offering or a sale of our company, given prevailing market conditions; |
| · | trends and developments in our industry; and |
| · | external market conditions affecting the life sciences and biotechnology industry sectors. |
Income Taxes. We recognize deferred income taxes for the future tax consequences attribute to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, operating loss and tax credit carryforwards. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the fiscal years in which those temporary differences are expected to be recovered or settled.
In accordance with Internal Revenue Code §382 (“IRC §382”), the future deductibility of our net operating losses (“NOLs”) may be subject to an annual limitation in the event of a change in control as defined by applicable regulations. We have yet to complete a formal study to confirm NOLs are not limited in utilization per IRC §382 and may reduce applicable deferred tax assets upon completion of such a study, in future periods.
The impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more likely than not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. We had no uncertain tax positions as of April 30, 2022.
Recent Accounting Pronouncements
See Note 3 to our financial statements included elsewhere in this report for additional information.
Emerging Growth Company Status
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act, until such time as those standards apply to private companies. We have elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that it (i) is no longer an emerging growth company or (ii) affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. As a result, these financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
| - 50 - |
Plan of Operations
Our plan of operations is currently focused on the development of both our therapeutic candidates which are at different stages of development. We submitted an IND application for AL001 to the FDA on June 30, 2021. On July 28, 2021, we announced receipt of FDA “Study May Proceed” letter for a Phase I study under our IND application for AL001, a lithium-based ionic cocrystal oral therapy for patients with dementia related to mild, moderate, and severe cognitive impairment associated with Alzheimer’s.
On August 17, 2021, we announced that we have contracted Altasciences Clinical Kansas (“Altasciences”) to conduct a six-month Phase I relative bioavailability study for AL001 for dementia related to Alzheimer’s beginning in September 2021. The Phase I first-in-human study is for the purpose of determining potential clinically safe and appropriate dosing for AL001 in future studies. The Phase I study investigated the pharmacokinetics (the movement of drug through the body) of lithium following a single dose of AL001 (the “study drug”) compared to a typical single dose of a marketed 300 mg immediate-release lithium carbonate capsule (the “comparator” – currently indicated to treat mood disorders) in healthy male and female subjects. The lithium and salicylate components of AL001 have been given within the amounts already approved for use in patients. The purpose of the research study is to test the safety, tolerability, and bioavailability (how much and when drug gets in the body) of the study drug, AL001, compared to the currently marketed formulation of the comparator, lithium carbonate. This was to ascertain what AL001 doses should be given, and how often, in subsequent Phase 2 safety and efficacy trials involving Alzheimer’s patients. At least 24 healthy male and female human subjects completed the Phase I trial.
On September 13, 2021, we announced that the first group of healthy participants have been dosed in a six-month Phase I relative bioavailability study for AL001 for dementia related to Alzheimer’s. On December 17, 2021, we announced that we received positive topline data from our Phase I clinical trial for AL001. A full report of the Phase I first-in-human study was completed in March 2022. The Phase I study was for the purpose of determining potential clinically safe and appropriate dosing for our ongoing Phase IIA MAD study. AL001 is a lithium-delivery system; it is a lithium-salicylate-L-proline engineered ionic co-crystal under development as an oral treatment for patients with dementia related to mild, moderate and severe cognitive impairment associated with Alzheimer’s.
We have an additional preclinical candidate for Alzheimer’s, AL002, which has transitioned from early-stage development to an extensive program of preclinical study and evaluation, which was completed on May 31, 2021 and was followed by a comprehensive report prepared by Charles River Laboratories, Inc., an independent preclinical service provider, received on July 23, 2021. Our preclinical program included a toxicologic evaluation, histopathology study and brain beta amyloid analysis and was expanded to include an immunoglobulin analysis and biodistribution study.
On July 30, 2021, we announced that we submitted a pre-IND meeting request for AL002 and supporting briefing documents to the Center for Biological Evaluation and Research of the FDA. On September 30, 2021, we announced that we have received a written response to our meeting request relating to our Type B Pre-IND application from the FDA providing a path for our planned clinical development of AL002. AL002 is a patented method using a mutant-peptide sensitized cell as a cell-based therapeutic vaccine that seeks to restore the ability of a patient’s immunological system to combat Alzheimer’s. Preclinical work supports AL002 being associated with a positive anti-inflammatory response and a decrease in brain amyloid contents. Based on AL002’s positive toxicology results, the biologic nature of this product and the urgent need to deliver treatments for Alzheimer’s to patients, we proposed, and the FDA agreed, to conduct a combined Phase I/II study.
We recently announced that the FDA’s agreement to us conducting a combined Phase I/II study, together with our process to identify the right manufacturing partner to provide our study drug materials for the Phase I/II study, has extended the timeline for when we anticipate filing the IND, which is now expected to be done in the third calendar quarter of 2022, and we plan to initiate the clinical trial of AL002 as soon as possible after the approval of the IND by the FDA.
On March 28, 2022, we announced receipt of full data set from Phase I clinical trial for AL001. The full data set builds upon topline data previously reported on December 17, 2021. These data affirmed that dose-adjusted relative bioavailability analyses of the rate and extent of lithium absorption in plasma indicate that AL001 at 150 mg dosage is bioavailability to the marketed 300 mg lithium carbonate product and the shapes of the lithium plasma concentration versus time curves are similar. AL001 salicylate plasma concentrations are observed to be well tolerated and consistently within safe limits and the safety profiles of both AL001 and the marketed lithium carbonate capsule were benign.
| - 51 - |
During Phase I first-in-human trial, participants received a single dose of AL001 containing lithium in an amount equivalent to 150 mg lithium carbonate; this is the dose proposed by the inventors as likely appropriate for Alzheimer’s treatment when given three times daily. Currently, marketed immediate-release lithium carbonate 300 mg are given three times daily; for example, lithium carbonate 300 mg three times daily is a dose commonly used for bipolar affective disorders. It can be difficult to set the appropriate dose of lithium carbonate and other lithium products due to the small margin between effective and toxic blood levels and to avoid side effects or inadequate treatment outcomes. We see the possibility of providing the benefits from lithium at up to 50% of the currently approved lithium carbonate dosage, with the potential for better outcomes and with elimination of the need for lithium therapeutic drug monitoring. Moreover, the data confirms AL001’s potential as a replacement of the current lithium-based treatments and may provide a treatment for over 40 million Americans suffering from Alzheimer’s and other neurodegenerative diseases and psychiatric disorders.
Such findings may allow us to design a development program that will potentially reduce the amount of new data generated to support approval. Bioequivalence may have utility for AL001 when seeking approval for the indications of currently marketed lithium products, and for new indications as a benchmark for safety. Given the systemic pharmacokinetic similarity to marketed immediate-release lithium carbonate products, AL001 is being dosed three times daily in the ongoing Phase IIA MAD study.
On April 4, 2022, we announced the appointment of Dr. Terri Hunter, Ph.D., a Technology Transfer Specialist, to our Scientific Advisory Board. During her tenure at the University of South Florida, Dr. Hunter was responsible for managing the patent portfolio associated with Alzamend’s two product candidates, AL001 and AL002.
On April 11, 2022, we announced that we contracted with Altasciences and iResearch Atlanta, LLC (“iResearch”) to manage and conduct, respectively, our Phase IIA MAD study in patients with mild to moderate Alzheimer’s. The Phase IIA study, which commenced enrollment in May 2022, is for the purposes of evaluating the safety and tolerability of AL001 under multiple dose, steady-state conditions, and to determine the maximum tolerated dose in patients with mild to moderate Alzheimer’s.
On April 28, 2022, we announced that DPL has made an additional investment in our company. On March 28, 2022, we announced receipt of the full data set from Phase I clinical trial for AL001. Based on the achievement of this milestone, under the March 12, 2021 securities purchase agreement, Alzamend sold an additional 2,666,667 shares of its common stock to DPL for $4 million, or $1.50 per share, and issued to DPL warrants to acquire 1,333,333 shares of its common stock with an exercise price of $3.00 per share.
On May 5, 2022, we announced that the first patient with mild to moderate Alzheimer’s has been dosed in a 12-month Phase IIA MAD study for dementia related to Alzheimer’s. The Phase IIA study will evaluate the safety and tolerability of AL001 under multiple-dose, steady-state conditions and determine the maximum tolerated dose in patients diagnosed with mild to moderate Alzheimer’s. Lithium has been well characterized for safety and is approved/marketed in multiple formulations for bipolar affective disorders. Lithium dosing for the MAD cohorts is based on a fraction of the usual dose for treatment of bipolar affective disorder (i.e., AL001 lithium content at a lithium carbonate equivalent of 300 mg three times daily, daily total of 900 mg), with the target dose for Alzheimer’s treatment at half of that lithium carbonate equivalent value (150 mg three times daily, daily total of 450 mg). In each cohort, consisting of six active and two placebo patients (as per randomization), multiple ascending doses will be administered three times daily for 14 days under fasted conditions (at least 1 hour before or 4 hours after meals) up to tolerability/safety limits. The lithium and salicylate components of AL001 will be given within the amounts already approved for use in patients. Up to 40 subjects will complete the Phase IIA trial. The maximum tolerated dose will then be used for further studies. Topline data are expected in December 2022 from this study.
On May 17, 2022, we announced that we have submitted a Pre-IND meeting request for AL001 and supporting briefing documents to the FDA for the treatment of bipolar disorder, MDD and PTSD. On July 18, 2022, we announced that we received a written response from the FDA. Based on the written response from the FDA, we plan to submit separate INDs for bipolar disorder, MDD, and PTSD after completion of the current Phase II MAD clinical trial, which would allow us to initiate Phase II studies in each of those indications.
The continuation of our current plan of operations with respect to completing our IND application and our series of human clinical trials for each of our therapeutics requires us to raise additional capital to fund our operations.
Because our working capital requirements depend upon numerous factors, including the progress of our preclinical and clinical testing, timing and cost of obtaining regulatory approvals, changes in levels of resources that we devote to the development of manufacturing and marketing capabilities, competitive and technological advances, status of competitors, and our ability to establish collaborative arrangements with other organizations, we will require additional financing to fund future operations.
| - 52 - |
Results of Operations
Results of Operations for the Year Ended April 30, 2022 Compared to Year Ended April 30, 2021
The following table summarizes the results of our operations for the years ended April 30, 2022 and 2021:
| For the Year Ended April 30, | ||||||||||||||||
| 2022 | 2021 | $ Change | % Change | |||||||||||||
| OPERATING EXPENSES | ||||||||||||||||
| Research and development | $ | 5,201,314 | $ | 1,310,716 | $ | 3,890,598 | 297 | % | ||||||||
| General and administrative | 7,118,221 | 3,641,172 | 3,477,049 | 95 | % | |||||||||||
| Total operating expenses | 12,319,535 | 4,951,888 | 7,367,647 | 149 | % | |||||||||||
| Loss from operations | (12,319,535 | ) | (4,951,888 | ) | (7,367647 | ) | 149 | % | ||||||||
| OTHER EXPENSE, NET | ||||||||||||||||
| Gain on extinguishment of debt | 4,000 | 62,418 | (58,418 | ) | -94 | % | ||||||||||
| Interest expense | (46,524 | ) | (142,421 | ) | 95,897 | -67 | % | |||||||||
| Interest expense - related party | - | (16,382 | ) | 16,382 | -100 | % | ||||||||||
| Interest income - related party | - | 1,706 | (1,706 | ) | -100 | % | ||||||||||
| Total other expense, net | (42,524 | ) | (94,679 | ) | 52,155 | -55 | % | |||||||||
| NET LOSS | $ | (12,362,059 | ) | $ | (5,046,567 | ) | $ | (7,315,492 | ) | 145 | % | |||||
| Basic and diluted net loss per common share | $ | (0.14 | ) | $ | (0.07 | ) | $ | (0.07 | ) | * | ||||||
| Basic and diluted weighted average common shares outstanding | 89,095,274 | 72,650,073 | * | |||||||||||||
| * | Not meaningful |
Revenue
We were formed on February 26, 2016 to acquire and commercialize patented intellectual property and know-how to prevent, treat and cure the crippling and deadly disease, Alzheimer’s. We currently have only two product candidates, AL001 and AL002. These products are in the preclinical stage of development and will require extensive clinical study, review and evaluation, regulatory review and approval, significant marketing efforts and substantial investment before either or both of them, and any respective successors, will provide us with any revenue. We did not generate any revenues during the years ended April 30, 2022 and 2021, and we do not anticipate that we will generate revenue for the foreseeable future.
Research and Development Expenses
Research and development expenses for the years ended April 30, 2022 and 2021 were $5.2 million and $1.3 million, respectively. As reflected in the table below, research and development expenses primarily consisted of professional fees, licenses and fees, as well as stock compensation expense:
| For the Year Ended April 30, | ||||||||||||||||
| 2022 | 2021 | $ Change | % Change | |||||||||||||
| Professional fees | $ | 3,869,032 | $ | 1,173,464 | $ | 2,695,568 | 230 | % | ||||||||
| Licenses and fees | 715,000 | 50,000 | 665,000 | 1330 | % | |||||||||||
| Stock compensation expense | 423,167 | 87,252 | 335,915 | 385 | % | |||||||||||
| Other research and development expenses | 194,115 | - | 194,115 | * | ||||||||||||
| Total research and development expenses | $ | 5,201,314 | $ | 1,310,716 | $ | 3,890,598 | 297 | % | ||||||||
| * Not meaningful | ||||||||||||||||
| - 53 - |
Professional Fees
During the years ended April 30, 2022 and 2021, we reported incurring professional fees of $3.9 million and $1.2 million, respectively, which were principally comprised of professional fees attributed to various types of scientific services, including FDA consulting services. The increase relates to professional fees incurred related to the Phase I study for AL001 for dementia related to Alzheimer’s.
Licenses and Fees
There are certain initial license fees and milestone payments required to be paid to the University of South Florida and the Licensor, for the licenses of the technologies, pursuant to the terms of the Standard Exclusive License Agreement with Sublicensing Terms.
During the year ended April 30, 2022, we incurred $715,000 in license fees related to completion of the Phase I study for AL001 for dementia related to Alzheimer’s. During the year ended April 30, 2021, we incurred $50,000 in license fees related to achieving the milestone of conducting pre-IND discussions with the FDA regarding AL001.
Stock Compensation Expense
During the years ended April 30, 2022 and 2021, we incurred $423,000 and $87,000, respectively, in research and development stock compensation expense related to stock option grants to consultants. All option grants are granted at the per share fair value on the grant date. Vesting of options differs based on the terms of each option. We valued the options at their date of grant utilizing the Black Scholes option pricing model. Stock-based compensation is a non-cash expense because we settle these obligations by issuing shares of our common stock from authorized shares instead of settling such obligations with cash payments.
General and Administrative Expenses
General and administrative expenses for the years ended April 30, 2022 and 2021 were $7.1 million and $3.6 million, respectively. As reflected in the table below, general and administrative expenses primarily consisted of the following expense categories: stock compensation expense; professional fees; insurances; as well as salaries and benefits. For the years ended April 30, 2022 and 2021, the remaining general and administrative expenses of $279,000 and $166,000, respectively, primarily consisted of payments for advertising and promotion, transfer agent fees, travel, and other office expenses, none of which is significant individually.
| For the Year Ended April 30, | ||||||||||||||||
| 2022 | 2021 | $ Change | % Change | |||||||||||||
| Stock compensation expense | $ | 3,985,403 | $ | 2,323,810 | $ | 1,661,593 | 72 | % | ||||||||
| Professional fees | 714,036 | 699,910 | 14,126 | * | ||||||||||||
| Insurance | 714,329 | - | 714,329 | 100 | % | |||||||||||
| Salary and benefits | 873,013 | 451,921 | 421,092 | 93 | % | |||||||||||
| Licenses and fees | 250,489 | - | 250,489 | 100 | % | |||||||||||
| Management services | 302,089 | - | 302,089 | 100 | % | |||||||||||
| Other general and administrative expenses | 278,862 | 165,531 | 113,331 | * | ||||||||||||
| Total general and administrative expenses | $ | 7,118,221 | $ | 3,641,172 | $ | 3,477,049 | 95 | % | ||||||||
| * | Not meaningful |
Stock Compensation Expense
During the years ended April 30, 2022 and 2021, we incurred general and administrative stock compensation expense of $4.0 million and $2.3 million, respectively, related to stock option grants to executives, employees and consultants as well as shares issued for services to Spartan Capital Securities, LLC (“Spartan Capital”). All option grants are granted at the per share fair value on the grant date. Vesting of options differs based on the terms of each option. We valued the options at their date of grant utilizing the Black Scholes option pricing model. We valued the shares issued for services at their intrinsic value on the date of issuance. Stock-based compensation is a non-cash expense because we settle these obligations by issuing shares of our common stock from authorized shares instead of settling such obligations with cash payments.
Salaries and Benefits
The second largest component of general and administrative expenses is salaries and benefits expense. During the years ended April 30, 2022 and 2021, we incurred $873,000 and $452,000, respectively, in employee-related expenses. As of April 30, 2022, we had four full-time and four part-time employees.
| - 54 - |
Insurance Expense
During the year ended April 30, 2022, we incurred insurance expense of $714,000, which was primarily directors and officers insurance that was required as part of the IPO process.
Professional Fees
During the years ended April 30, 2022 and 2021, we reported professional fees of $714,000 and $700,000, respectively, which were principally comprised of the following items:
Year Ended April 30, 2022
| · | In June 2017, we entered into a five-year consulting agreement with Spartan Capital pursuant to which Spartan Capital agreed to provide consulting services with respect to general corporate matters. In December 2017, we paid to Spartan Capital a consulting fee of $1.4 million for the services to be rendered over the 60-month term of this consulting agreement. During the year ended April 30, 2022, we recorded an expense of $248,000 as a result of this consulting agreement. |
| · | During the year ended April 30, 2022, we incurred $249,000 in audit and tax fees, $89,000 in legal fees, $88,000 in related party consulting and $40,000 in investor relations expenses. |
Year Ended April 30, 2021
| · | During the year ended April 30, 2021, we recorded an expense of $280,000 in connection with the five-year consulting agreement with Spartan Capital. |
| · | In June 2019, we entered into a two-year uplisting agreement (the “Uplisting Agreement”) with Spartan Capital pursuant to which Spartan Capital agreed to provide consulting services with respect to a potential public offering. Compensation under this agreement consisted of a cash payment in the amount of $475,000 and the issuance of 500,000 shares of common stock. We are amortizing the cost of these services over the two-year term of the Uplisting Agreement. During the year ended April 30, 2021, we recorded an expense of $263,000 in connection with the Uplisting Agreement. The Uplisting Agreement was terminated on March 3, 2021. |
| · | During the year ended April 30, 2021, we incurred $107,000 in audit fees, $26,000 in legal fees and $24,000 in investor relations expenses. |
Other Expense, Net
Gain on Extinguishment of Debt
In May 2020, we received an advance of $4,000 and loan proceeds in the amount of $62,000 under the Paycheck Protection Program (“PPP”). The PPP, established as part of the Coronavirus Aid, Relief and Economic Security Act (“CARES Act”), provides for loans to qualifying businesses for amounts up to 2.5 times of the average monthly payroll expenses of the qualifying business. The loans and accrued interest are forgivable after the earlier of (i) 24 weeks after the loan disbursement date and (ii) December 31, 2020; as long as the borrower uses the loan proceeds for eligible purposes, including payroll, benefits, rent and utilities, and maintains its payroll levels.
We used the proceeds for purposes consistent with the PPP. In December 2020, we met the conditions and received forgiveness of the advance of $4,000 and loan of $62,000 and recorded the benefit as a gain on extinguishment of debt.
Interest Expense
Interest expense was $47,000 for the year ended April 30, 2022 related to the convertible promissory note issued in August 2020, including non-cash interest expense of $13,000 recorded from the amortization of debt discount. Interest expense was $142,000 for the year ended April 30, 2021 related to the convertible promissory note issued in August 2020, including non-cash interest expense of $124,000 recorded from the amortization of debt discount.
| - 55 - |
Current and Deferred Income Taxes
As of April 30, 2022 and 2021, we had deferred tax assets totaling $10.1 million and $4.4 million, respectively. The ultimate realization of deferred tax assets is dependent upon the existence, or generation, of taxable income in the periods when those temporary differences and net operating loss carryovers are deductible. Management considers the scheduled reversal of deferred tax liabilities, taxes paid in carryover years, projected future taxable income, available tax planning strategies, and other factors in making this assessment. Based on available evidence, management believes it is less likely than not that all of the deferred tax assets will be realized. Accordingly, we have established a 100% valuation allowance. As a result of the full valuation allowance, we did not record an income tax benefit during the years ended April 30, 2022 and 2021.
Liquidity and Capital Resources
The accompanying financial statements have been prepared on the basis that our company will continue as a going concern. As of April 30, 2022, we had cash of $14.1 million and an accumulated deficit of $29.2 million. We have incurred recurring losses and reported losses for the year ended April 30, 2022 totaling $12.4 million. In the past, we have financed our operations principally through sales of promissory notes and equity securities.
In March of 2021, we entered into a securities purchase agreement with DPL, pursuant to which we agreed to sell an aggregate of 6,666,667 shares of common stock for an aggregate of $10 million, or $1.50 per share, which sales will be made in tranches. On March 9, 2021, DPL paid $4 million, less the $1.8 million in prior advances and the surrender for cancellation of the $50,000 convertible promissory note, previously issued to BitNile, for an aggregate of 2,666,667 shares of common stock. Under the terms of the securities purchase agreement, DPL (i) purchased, in July 2021, an additional 1,333,333 shares of common stock upon FDA approval of our IND for our Phase I clinical trials for AL001 for a purchase price of $2 million; and (ii) on April 26, 2022, purchase 2,666,667 shares of common stock upon completion of these Phase I clinical trials for AL001 for a purchase price of $4 million. In addition, we issued DPL warrants to purchase an aggregate of 6,666,667 shares of common stock at an exercise price of $3.00 per share. Finally, we agreed that for a period of 18 months following the date of the payment of the final tranche of $4 million on April 26, 2022, DPL will have the right to invest an additional $10 million on the same terms, except that no specific milestones have been determined with respect to the additional $10 million as of the date of this Annual Report.
On June 17, 2021, we announced the closing of our IPO of 2,875,000 shares of common stock at a price to the public of $5.00 per share. The proceeds from the offering to us, net of underwriting discounts and commissions and offering expenses, were approximately $12.9 million. Our common stock is listed on The Nasdaq Capital Market under the ticker symbol “ALZN”.
We will need to obtain substantial additional funding in the future for our clinical development activities and continuing operations. If we are unable to raise capital when needed or on favorable terms, we would be forced to delay, reduce, or eliminate our research and development programs or future commercialization efforts. Our future capital requirements will depend on many factors, including:
| · | successful enrollment in and completion of clinical trials; |
| · | our ability to establish agreements with third-party manufacturers for clinical supply for our clinical trials and, if our product candidates are approved, commercial manufacturing; |
| · | our ability to maintain our current research and development programs and establish new research and development programs; |
| · | addition and retention of key research and development personnel; |
| · | our efforts to enhance operational, financial, and information management systems, and hire additional personnel, including personnel to support development of our product candidates; |
| · | negotiating favorable terms in any collaboration, licensing, or other arrangements into which we may enter and performing our obligations in such collaborations; |
| · | the timing and amount of milestone and other payments we may receive under our collaboration arrangements; |
| · | our eventual commercialization plans for our product candidates; |
| · | the costs involved in prosecuting, defending, and enforcing patent claims and other intellectual property claims; and |
| · | the costs and timing of regulatory approvals. |
A change in the outcome of any of these or other variables with respect to the development of any of our product candidates could significantly change the costs and timing associated with the development of that product candidate. Furthermore, our operating plans may change in the future, and we may need additional funds to meet operational needs and capital requirements associated with such operating plans.
We expect to continue to incur losses for the foreseeable future and need to raise additional capital until we are able to generate revenues from operations sufficient to fund our development and commercial operations. However, based on our current business plan, we believe that our cash and cash equivalents at April 30, 2022, are sufficient to meet our anticipated cash requirements during the twelve-month period subsequent to the issuance of the financial statements included in this Annual Report.
| - 56 - |
Cash Flows
The following table summarizes our cash flows for the years ended April 30, 2022 and 2021:
| For the Year Ended April 30, | ||||||||
| 2022 | 2021 | |||||||
| Net cash provided by (used in): | ||||||||
| Operating activities | $ | (6,613,990 | ) | $ | (2,712,027 | ) | ||
| Investing activities | (106,458 | ) | 100,915 | |||||
| Financing activities | 18,854,989 | 4,450,097 | ||||||
| Net increase in cash and cash equivalents | $ | 12,134,541 | $ | 1,838,985 | ||||
Operating Activities
During the year ended April 30, 2022, net cash used in operating activities was $6.6 million. This consisted primarily of a net loss of $12.4 million, partially offset by non-cash charges of $4.4 million in stock-based compensation expense and an increase in our net operating assets and liabilities of $1.3 million. The increase in our net operating assets and liabilities was due to an increase in accounts payable and accrued expenses and a decrease in prepaid expenses and other current assets. Prepaid expenses decreased primarily from the amortization of Spartan Capital consulting fees in the amount of $280,000 and offering costs in the amount of $353,000.
During the year ended April 30, 2021, net cash used in operating activities was $2.7 million. This consisted primarily of a net loss of $5.0 million, offset by non-cash charges of $2.4 million in stock-based compensation expense and a decrease in our net operating assets and liabilities of $152,000. The decrease in our net operating assets and liabilities was due to a decrease in accounts payable and accrued expenses and an increase in prepaid expenses and other current assets.
Investing Activities
During the year ended April 30, 2022, net cash used in investing activities was $106,000, from the purchase of equipment and machinery. We purchased a CliniMACS Plus instrument to be used on the ALZN002 project at the University of Miami. The machine was purchased from Miltenyi Biotec and is utilized to separate the monocytes from blood. We purchased this equipment to streamline the development of DCs to create the AL002 vaccine for patients in the 24-months Phase I/II clinical trials.
During the year ended April 30, 2021, net cash provided by investing activities was $101,000. This consisted of proceeds from repayment of notes receivable from Avalanche, a related party. In August 2020, the principal and accrued interest on the AVLP Note was paid in full.
Financing Activities
During the year ended April 30, 2022, net cash provided by financing activities was $18.9 million. This consisted primarily of proceeds from our initial public offering of $12.9 million, net of costs, and proceeds of $6 million from the issuance of common stock and warrants to DPL. On July 28, 2021, we received from the FDA a “Study May Proceed” letter for a Phase IA study under our IND application for AL001. Based on the achievement of this milestone, we sold an additional 1,333,333 shares of common stock to DPL for $2 million, or $1.50 per share, and issued to DPL warrants to acquire 666,667 shares of our common stock with an exercise price of $3.00 per share. On March 28, 2022, we a received the full data set from the Phase I clinical trial for AL001. Based on the achievement of this milestone, we sold an additional 2,666,667 shares of our common stock to DPL for $4.0 million, or $1.50 per share, and issued to DPL warrants to acquire 1,333,333 shares of our common stock with an exercise price of $3.00 per share.
During the year ended April 30, 2021, net cash provided by financing activities was $4.5 million. This consisted primarily of proceeds from the issue of common stock and short-term advances from DPL.
Contractual Obligations
On May 1, 2016, we entered into a Standard Exclusive License Agreement for AL002 with Sublicensing Terms with the Licensor, pursuant to which the Licensor granted us a royalty bearing exclusive worldwide license limited to the field of Alzheimer’s Immunotherapy and Diagnostics, under United States Patent No. 8,188,046, entitled “Amyloid Beta Peptides and Methods of Use,” filed April 7, 2009 and granted May 29, 2012.
| - 57 - |
There are certain initial license fees and milestone payments required to be paid by us to the Licensor, pursuant to the terms of license agreements we have entered into with the Licensor. The license agreements for AL002 require us to pay royalty payments of 4% on net sales of products developed from the licensed technology for AL002 while the license agreements for AL001 require that we pay combined royalty payments of 4.5% on net sales of products developed from the licensed technology for AL001. We have already paid an initial license fee of $200,000 for AL002 and an initial license fee of $200,000 for AL001. As an additional licensing fee for the license of AL002, the Licensor received 3,601,809 shares of our common stock. As an additional licensing fee for the license of the AL001 technologies, the Licensor received 2,227,923 shares of our common stock. Minimum royalties for AL001 are $25,000 in 2023, $45,000 in 2024 and $70,000 in 2025 and every year thereafter, for the life of the agreement. Minimum royalties for AL002 are $20,000 in 2022, $40,000 in 2023 and $50,000 in 2024 and every year thereafter, for the life of the respective agreement. Additionally, we are required to pay milestone payments on the due dates to the Licensor for the license of the AL001 technologies and for the AL002 technology, as follows:
Original AL001 License:
| Payment | Due Date | Event | |||
| $ | 50,000 | * | Completed September 2019 | Pre-IND meeting | |
| $ | 65,000 | * | Completed June 2021 | ND application filing | |
| $ | 190,000 | * | Completed December 2021 | Upon first dosing of patient in a clinical trial | |
| $ | 500,000 | * | Completed March 2022 | Upon Completion of first clinical trial | |
| $ | 1,250,000 | 12 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 10,000,000 | 8 years from the effective date of the agreement | Upon FDA approval | ||
*Milestone met and completed
AL002 License:
| Payment | Due Date | Event | |||
| $ | 50,000 | * | Upon IND application filing | Upon IND application filing | |
| $ | 50,000 | 12 months from IND application filing date | Upon first dosing of patient in first Phase I clinical trial | ||
| $ | 175,000 | 12 months from first patient dosed in Phase I | Upon completion of first Phase I clinical trial | ||
| $ | 500,000 | 24 months from completion of first Phase I clinical trial | Upon completion of first Phase II clinical trial | ||
| $ | 1,000,000 | 12 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 10,000,000 | 7 years from the effective date of the agreement | Upon FDA BLA approval | ||
*Milestone met and completed
We have met the pre-IND meeting, IND application filing, and successfully completed the Phase I clinical trial milestones encompassing AL001. If we fail to meet a milestone by its specified date, Licensor may terminate the license agreement.
The Licensor was also granted a preemptive right to acquire such shares or other equity securities that may be issued from time to time by us while the Licensor remains the owner of any equity securities of our company.
On June 10, 2020, we obtained two (2) additional royalty-bearing exclusive worldwide licenses from the Licensor to a therapy named AL001. One of the additional licenses is for the treatment of neurodegenerative diseases excluding Alzheimer’s and the other license is for the treatment of psychiatric diseases and disorders. There are certain license fees and milestone payments required to be paid pursuant to the terms of the June AL001 License Agreements. Under each of the June AL001 License Agreements, a royalty payment of 3% is required on net sales of products developed from the licensed technology. For the two additional AL001 licenses, in the aggregate, we paid initial license fees of $20,000. Additionally, under each of the June AL001 License Agreements, we are required to pay milestone payments on the due dates to the Licensor for the license of the technology, as follows:
| - 58 - |
Additional AL001 Licenses:
| Payment | Due Date | Event | |||
| $ | 50,000 | Upon IND application filing | IND application filing | ||
| $ | 150,000 | 12 months from IND filing date | Upon first dosing of patient in a clinical trial | ||
| $ | 400,000 | 12 months from first patient dosing | Upon Completion of first clinical trial | ||
| $ | 1,000,000 | 36 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 8,000,000 | 8 years from the effective date of the agreement | First commercial sale | ||
Recent Accounting Standards
For information about recent accounting pronouncements that may impact our financial statements, please refer to Note 3 of Notes to Financial Statements under the heading “Recent Accounting Standards.”
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Because we are a smaller reporting company, this section is not applicable.
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The financial statements required by this Item 8 are included in this Annual Report following Item 16 hereof. As a smaller reporting company, we are not required to provide supplementary financial information.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES |
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our periodic and current reports that we file with the SEC is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can provide only reasonable and not absolute assurance of achieving the desired control objectives. In reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. In addition, the design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
As of April 30, 2022, we carried out an evaluation, under the supervision of, and with the participation of, our management, including our principal executive officer and principal financial officer, of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Rule 13a-15(b) under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). We have established disclosure controls and procedures designed to ensure that information required to be disclosed in the reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms and is accumulated and communicated to management, including the principal executive officer and principal financial officer, to allow timely decisions regarding required disclosure.
Based upon that evaluation, our principal executive officer and principal financial officer, with the assistance of other members of the Company's management, have evaluated the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of the end of the period covered by this annual report and has determined that our disclosure controls and procedures were not effective due to the material weaknesses as described herein.
| - 59 - |
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Our internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that: (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management assessed the effectiveness of our internal control over financial reporting as of April 30, 2022. In making this assessment, our management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control-Integrated 2013 Framework. Our management has concluded that, as of April 30, 2022, our internal control over financial reporting was not effective.
A material weakness is a control deficiency (within the meaning of the Public Company Accounting Oversight Board (United States) Auditing Standard No. 2) or combination of control deficiencies that result in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected. Management has identified the following material weaknesses:
| 1. | We do not have sufficient resources in our accounting function, which restricts our ability to perform sufficient reviews and approval of manual journal entries posted to the general ledger and to consistently execute review procedures over general ledger account reconciliations, financial statement preparation and accounting for non-routine transactions; and |
| 2. | Our primary user access controls (i.e., provisioning, de-provisioning, privileged access and user access reviews) to ensure appropriate authorization and segregation of duties that would adequately restrict user and privileged access to the financially relevant systems and data to appropriate personnel were not designed and/or implemented effectively. We did not design and/or implement sufficient controls for program change management to certain financially relevant systems affecting our processes. |
Planned Remediation
We are implementing measures designed to improve our internal control over financial reporting to remediate material weaknesses, including the following:
| · | Formalizing our internal control documentation and strengthening supervisory reviews by our management; and |
| · | Adding additional accounting personnel and segregating duties amongst accounting personnel. |
Management continues to work to improve its controls related to our material weaknesses, specifically relating to user access and change management surrounding our information technology systems and applications. Management will continue to implement measures to remediate material weaknesses, such that these controls are designed, implemented, and operating effectively. The remediation actions include: (i) enhancing design and documentation related to both user access and change management processes and control activities; and (ii) developing and communicating additional policies and procedures to govern the area of information technology change management. In order to achieve the timely implementation of the above, management has commenced the following actions and will continue to assess additional opportunities for remediation on an ongoing basis:
| · | Engaging a third-party specialist to assist management with improving the Company’s overall control environment, focusing on change management and access controls; and |
| · | Implementing new applications and systems that are aligned with management’s focus on creating strong internal controls. |
| - 60 - |
We are currently working to improve and simplify our internal processes and implement enhanced controls, as discussed above, to address the material weaknesses in our internal control over financial reporting and to remedy the ineffectiveness of our disclosure controls and procedures. These material weaknesses will not be considered to be remediated until the applicable remediated controls are operating for a sufficient period of time and management has concluded, through testing, that these controls are operating effectively.
Despite the existence of these material weaknesses, we believe that the consolidated financial statements included in the period covered by this Annual Report on Form 10-K fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented in conformity with U.S. generally accepted accounting principles.
Changes in Internal Control over Financial Reporting
During the fourth fiscal quarter of 2022, there were no changes in our internal control over financial reporting which were identified in connection with management’s evaluation required by paragraph (d) of Rules 13a-15 and 15d-15 under the Exchange Act, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
None.
| ITEM 9C. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
Not applicable.
| - 61 - |
PART III
| ITEM 10. | Directors, Executive Officers and Corporate Governance |
The following table sets forth the names and ages of our executive officers, directors and director nominees, and their positions with us, as of the date of this Annual Report:
| Name | Age | Position | ||
| Stephan Jackman | 46 | Chief Executive Officer and Director | ||
| Henry C.W. Nisser | 53 | Executive Vice President, General Counsel and Director | ||
| Kenneth S. Cragun | 61 | Senior Vice President of Finance | ||
| David J. Katzoff | 60 | Chief Operating Officer | ||
| Lien T. Escalona | 53 | Chief Financial Officer | ||
| William B. Horne | 54 | Chairman of the Board | ||
| Mark Gustafson | 62 | Director | ||
| Lynne Fahey McGrath, M.P.H., Ph.D. | 67 | Director | ||
| Jeffrey Oram | 55 | Director | ||
| Andrew H. Woo, M.D., Ph.D. | 59 | Director |
The following information provides a brief description of the business experience of each executive officer and director.
Stephan Jackman joined our company as Chief Executive Officer in November 2018. Mr. Jackman was elected as a director in September 2020. He has played an intricate role in the development of therapeutic treatments, products and programs from the research stage to market and commercialization. Mr. Jackman has demonstrated a dedicated dual focus of creating value for internal and external stakeholders while developing strategic alliances and cross-function teams to meet and exceed goals. Prior to joining our company, from October 2017 to November 2018, Mr. Jackman was the Chief Operating Officer of Ennaid Therapeutics, an emerging biopharmaceutical company focusing on cures for mosquito borne infectious diseases such as Zika and Dengue viruses. From October 2015 to October 2017, Mr. Jackman was Chief Operating Officer of Exit 9 Technologies, a technology startup with a digital platform that connects retailers, publishers and customers. Additionally, from August 2014 to October 2015, he was an independent project and management consultant assisting startups, Fortune 500 companies and non-profits with major strategic initiatives. He has also held positions of increasing responsibility at Novartis Pharmaceuticals Corporation, L’Oréal USA, SBM Management Services and Family Intervention Services. Mr. Jackman holds a Master of Science in Management and a Bachelor of Engineering in Mechanical Engineering from Stevens Institute of Technology. Mr. Jackman’s 15 years of experience in life sciences and growth companies, day-to-day operational leadership of our company and in-depth knowledge of our drug candidates make him well qualified as a member of the Board.
Henry C.W. Nisser has served as our Executive Vice President and General Counsel on a part-time basis since May 2019. Mr. Nisser was appointed as a director in September 2020. Since May 2019, Mr. Nisser has served as the Executive Vice President and General Counsel of BitNile and as one of its directors since September 2020; he became BitNile’s President on January 12, 2021. Since February 2021, Mr. Nisser has served as the President, General Counsel and a director of Ault Disruptive Technologies Corporation, a publicly traded special purpose acquisition company (“Ault Disruptive”). Mr. Nisser is the Executive Vice President and General Counsel of Avalanche. From October 2011 through April 2019, Mr. Nisser was an associate and subsequently a partner with Sichenzia Ross Ference LLP, a law firm in New York. While with this law firm, his practice was concentrated on national and international corporate law, with a particular focus on U.S. securities compliance, public as well as private M&A, equity and debt financings and corporate governance. Mr. Nisser drafted and negotiated a variety of agreements related to reorganizations, share and asset purchases, indentures, public and private offerings, tender offers and going private transactions. Mr. Nisser is fluent in French and Swedish, as well as conversant in Italian. Mr. Nisser received his B.A. degree from Connecticut College, where he majored in International Relations and Economics. He received his LL.B. from University of Buckingham School of Law in the United Kingdom. We believe that Mr. Nisser’s extensive legal experience involving complex transactions and comprehensive knowledge of securities laws and corporate governance requirements applicable to listed companies give him the qualifications and skills to serve as one of our directors.
| - 62 - |
Kenneth S. Cragun joined our company on a part-time basis in December 2018. Since February 2021, Mr. Cragun has served as the Chief Financial Officer of Ault Disruptive. Since August 2020, Mr. Cragun has served as the Chief Financial Officer of BitNile and between October 2018 and August 2020, served as its Chief Accounting Officer. Since September 2018, Mr. Cragun has served on the board of directors and Chairman of the Audit Committee of Verb Technology Company, Inc. He served as a CFO Partner at Hardesty, LLC, a national executive services firm between October 2016 and October 2018. His assignments at Hardesty included serving as Chief Financial Officer of CorVel Corporation, a publicly traded company and a nationwide leader in technology driven, healthcare-related, risk management programs, and of RISA Tech, Inc., a private structural design and optimization software company. Mr. Cragun was also Chief Financial Officer of two Nasdaq-traded companies, Local Corporation, from April 2009 to September 2016, which operated Local.com, a U.S. top 100 website, and Modtech Holdings, Inc., from June 2006 to March 2009, a supplier of modular buildings. Prior thereto, he had financial leadership roles with increasing responsibilities at MIVA, Inc., ImproveNet, Inc., NetCharge Inc., C-Cube Microsystems, Inc, and 3-Com Corporation. Mr. Cragun began his professional career at Deloitte. Mr. Cragun holds a Bachelor of Science degree in accounting from Colorado State University-Pueblo.
David J. Katzoff joined our company on a part-time basis in November 2019, serving as our Senior Vice President of Operations from November 2019 to December 2020, and currently serves as our Chief Operating Officer since December 2020. Mr. Katzoff has served as Senior Vice President of Finance of BitNile since January 2019. Since December 2021, Mr. Katzoff has served as the Chief Financial Officer of Imperalis Holding Corp., a publicly listed company. Since February 2021, Mr. Katzoff has served as the Vice President of Finance of Ault Disruptive. From 2015 to 2018, Mr. Katzoff served as Chief Financial Officer of Lumina Media, LLC, a privately-held media company and publisher of life-style publications. From 2003 to 2017, Mr. Katzoff served a Vice President of Finance of Local Corporation, a publicly-held local search company. Mr. Katzoff received a B.S. degree in Business Management from the University of California at Davis.
Lien T. Escalona joined our company as our full-time Chief Financial Officer in June 2021. She had served as the Director of Reporting on a part-time basis at BitNile from January to May 2021. Previously, Ms. Escalona was the Director of Financial Reporting for Confie Seguros Holding Co. from June to December 2020 and Landsea Homes Corporation from January 2019 to June 2020, where she was involved in the companies’ special purpose acquisition company, or SPAC, transactions. From February to December 2018, Ms. Escalona served as the acting Director of Business Acquisitions for Smilebrands, Inc., a healthcare company, working on acquisitions and purchase price accounting matters. From March 2015 to January 2018, Ms. Escalona served as an independent contractor to Western Digital Corporation in several capacities, ranging from financial reporting, SEC reporting, systems implementation, purchase price accounting, to training and cross-training. Ms. Escalona has served as an independent accounting contractor to various public companies in the Silicon Valley, Los Angeles and Orange County areas for more than 25 years in multiple industries, with an emphasis on accounting and finance, system implementation and SEC reporting. Ms. Escalona received a B.A. degree in Social Ecology from the University of California, Irvine.
William B. Horne has served as a director of our company since June 2016 and upon the effectiveness of our initial public offering in June 2021, Mr. Horne become our Chairman of the Board. Mr. Horne served as our Chief Financial Officer from June 2016 through December 2018. Mr. Horne has been a member of the board of directors of BitNile since October 2016. In January 2018, Mr. Horne was appointed as BitNile’s Chief Financial Officer until August 2020, when he resigned as its Chief Financial Officer and was appointed as its President. On January 12, 2021, Mr. Horne resigned as BitNile’s President and became its Chief Executive Officer. Mr. Horne has served as a director and Chief Executive Officer of Ault Disruptive Technologies Corporation, a special purpose acquisition company, since its inception in February 2021. Mr. Horne has served as a director and Chief Financial Officer of Avalanche since June 2016. Mr. Horne has served as a director and Chief Financial Officer of Ault & Co. since October 2017. Mr. Horne previously held the position of Chief Financial Officer in various public and private companies in the healthcare and high-tech field. Mr. Horne has a Bachelor of Arts Magna Cum Laude in Accounting from Seattle University. We believe that Mr. Horne's extensive financial and accounting experience in diversified industries and with companies involving complex transactions give him the qualifications and skills to serve as one of our directors.
Mark Gustafson joined our Board of Directors and became the Chairman of the Audit Committee in June 2021. Mr. Gustafson is a Chartered Professional Accountant with over 35 years of corporate, private and public company experience. Since April 2021, Mr. Gustafson has been the Chief Financial Officer, and since January 2022, a director, for PharmaKure Limited, a private London-based biopharmaceutical company dedicated to the treatment of neurodegenerative diseases. Since December 2021, Mr. Gustafson has served as an independent director and Chairman of the Audit Committee of Ault Disruptive. Since June 2020, Mr. Gustafson has served as the founder and director of Alpha Helium Inc., a private Canadian-based company helium exploration company. From 2014 to 2020, he was the Chief Executive Officer of Challenger Acquisitions Limited, a London Stock Exchange listed entertainment company. From 2010 to 2012, Mr. Gustafson was the President and Chief Executive Officer of Euromax Resources Limited, a Toronto Stock Exchange listed mineral exploration company. From 2005 to 2009, he served as Chairman and Chief Executive Officer of Triangle Energy Corporation, a New York Stock Exchange listed oil and gas exploration company, from 2004 to 2006, he served as President and Chief Executive Officer of Torrent Energy Corporation, a private oil and gas company, and from 2001 to 2002, he served as a financial consultant for Samson Oil & Gas and Peavine Resources, two private oil and gas companies. From 1997 to 1999, Mr. Gustafson served as President and Chief Executive Officer of Total Energy Services Ltd., a Toronto Stock Exchange listed oilfield services company, from 1993 to 1995, he served as the Chief Financial Officer of Q/media Software Corporation, a Toronto Stock Exchange listed software company, and from 1987 to 1993, he served initially as the Chief Financial Officer and then as a Vice President in charge of two operating divisions at EnServ Corporation, a Toronto Stock Exchange listed oilfield services company. From 1981 to 1987, he served as an audit manager at Price Waterhouse in Calgary Alberta. Mr. Gustafson received his Bachelor of Business Administration from Wilfrid Laurier University. Mr. Gustafson has been a Chartered Accountant since 1983. We believe that Mr. Gustafson’s over 35 years of corporate, private and public company operational and financial experience gives him the qualifications and skills to serve as one of our directors and as Chairman of the Audit Committee.
| - 63 - |
Lynne Fahey McGrath, M.P.H., Ph.D. joined our Board of Directors in June 2021. Dr. McGrath has served as a consultant to various companies in the biopharmaceutical industry, including: to the executive team of Nobias Therapeutics, Inc., a biotechnology product development company, between May 2020 and December 2021; a regulatory consultant with FoxKiser, LLC, a biotechnology consulting firm, from August 2018 to March 2020; and a regulatory consultant with Catalyst Healthcare Consulting, a biotechnology consulting firm, from 2020 to 2021. Dr. McGrath was a senior lead and Vice President of Regulatory Affairs at Regenxbio, Inc., where she headed global strategy for its portfolio of gene therapy products, from April 2015 to July 2018. Previously, she held senior positions at Novartis Corporation including Vice President, Global Head of Regulatory Affairs at Novartis Consumer Health and U.S. Head of Regulatory Affairs at Novartis Oncology from 2003 to April 2015. Dr. McGrath received a B.S. degree from the University of Connecticut, M.S. in Environmental Science from Rutgers University and M.P.H. and Ph.D. in Public Health from the University of Medicine and Dentistry of New Jersey Robert Wood Johnson Medical School. We believe that Dr. McGrath’s expertise in regulatory affairs and pharmaceutical product development across a range of therapeutic categories and her more than 30 years of experience directing worldwide approvals of more than 50 new drugs and indications makes her well qualified to serve as one of our directors.
Jeffrey Oram joined our Board of Directors in June 2021. Mr. Oram is a business professional with more than 25 years of corporate, private and institutional investment experience. Mr. Oram has spent the last 13 years in the institutional real estate capital markets. Since 2016, he has been a Principal at Godby Realtors, a private real estate investment and brokerage firm. From 2010 to 2018, Mr. Oram served as an Executive Member of the New Jersey State Investment Council, which oversees the investment of the State of New Jersey’s pension fund. From 2011 to 2016, he served as Executive Managing Director at Colliers International, from 2009 to 2011 he served as Director at Marcus and Millichap, and from 2003 to 2009, served as First Vice President at CB Richard Ellis. Mr. Oram received a Bachelor of Science degree in Biology from Princeton University. We believe that Mr. Oram’s 25 years of corporate, private and institutional investment experience gives him the qualifications and skills to serve as one of our directors.
Andrew H. Woo, M.D., Ph.D. joined our Board of Directors in June 2021. Dr. Woo is in private practice at Santa Monica Neurological Consultants and serves as an Assistant Clinical Professor of Neurology at the David Geffen School of Medicine at UCLA and Cedars-Sinai Medical Center. He also serves on the board for the Multiple Sclerosis Association of America and its Navigating MS International Steering Committee. He has been presented with UCLA clinical faculty teaching awards in 2006, 2012 and 2019 and is listed in America’s Top Physicians by the Consumer Research Council of America and Castle Connolly America’s Top Doctors 2006, 2007, 2010-2021, Southern California Super Doctors since 2008, and Los Angeles Magazine Top Doctors. He is an invited speaker at the Muntada International Symposium in Abu Dhabi. Dr. Woo received his B.A. from Cornell University and completed his M.D. and Ph.D. in Neuroimmunology in the Department of Molecular and Cell Biology at Brown University. He completed his medicine internship at Weil-Cornell Presbyterian Hospital/Cornell Medical Center in New York, his neurology residency at UCLA, and his fellowship in neurophysiology at Harbor-UCLA. We believe that Dr. Woo’s extensive medical experience gives him the qualifications and skills and relevant insight to serve as one of our directors.
Board Leadership Structure and Risk Oversight
Our Board is currently chaired by Mr. Horne. Mr. Horne has been a director since June 2016 and served as our Chief Financial Officer from June 2016 until December 2018. Given Mr. Horne’s extensive history with and knowledge of our company, we believe his role as our Chairman facilitates a regular flow of information between the Board and management and ensures that they both act with a common purpose.
One of the key functions of our Board is informed oversight of our risk management process. Our Board does not have a standing risk management committee, but rather administers this oversight function directly through the Board as a whole, as well as through various standing committees of our Board that address risks inherent in their respective areas of oversight. In particular, our Board is responsible for monitoring and assessing strategic risk exposure, including a determination of the nature and level of risk appropriate for us. Our Audit Committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The Audit Committee also monitors compliance with legal and regulatory requirements, in addition to oversight of the performance of our internal audit function. Our Nominating and Corporate Governance Committee monitors the effectiveness of our corporate governance guidelines, including whether they are successful in preventing illegal or improper liability-creating conduct. Our Compensation Committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
| - 64 - |
Term of Office
Directors serve until the next annual meeting of our stockholders and until their successors are elected and qualified. Officers are appointed to serve at the discretion of our Board of Directors.
Family Relationships
There are no family relationships among any of our executive officers and directors.
Involvement in Certain Legal Proceedings
Except as set forth below, to the best of our knowledge, during the past 10 years, none of the following occurred with respect to a present or former director, executive officer or employee:
| • | been convicted in a criminal proceeding or been subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| • | had any bankruptcy petition filed by or against the business or property of the person, or of any partnership, corporation or business association of which he was a general partner or executive officer, either at the time of the bankruptcy filing or within two years prior to that time; |
| • | been subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction or federal or state authority, permanently or temporarily enjoining, barring, suspending or otherwise limiting, his involvement in any type of business, securities, futures, commodities, investment, banking, savings and loan, or insurance activities, or to be associated with persons engaged in any such activity; |
| • | been found by a court of competent jurisdiction in a civil action or by the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| • | been the subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated (not including any settlement of a civil proceeding among private litigants), relating to an alleged violation of any federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; and |
| • | or been the subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Mr. Cragun served as Chief Financial Officer of Local Corporation (April 2009 to September 2016), which, in June 2015, filed a voluntary petition in the U.S. Bankruptcy Court for the Central District of California seeking relief under the provisions of Chapter 11 of Title 11 of the United States Code.
Except as disclosed in “Certain Relationships and Related Party Transactions,” none of our directors or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Code of Business Conduct and Ethics
Our Board has adopted a written code of business conduct and ethics, revised effective May 25, 2021, that applies to our directors, officers and employees, including our principal executive officer, principal financial officer and principal accounting officer or controller, or persons performing similar functions (the “Code of Conduct and Ethics”). In addition, on May 25, 2021, we adopted Code of Ethics for our Chief Executive Officer and our Senior Financial Officers (the “Code of Ethics”). We have posted on our website a current copy of both codes and all disclosures that are required by law in regard to any amendments to, or waivers from, any provision of the Code of Conduct and Ethics.
| - 65 - |
Director Independence
We use the definition of “independence” of the Nasdaq Marketplace Rules to make this determination. Rule 5605(a)(2) of the Nasdaq Marketplace Rules provides that an “independent director” is a person other than an officer or employee of the company or any other individual having a relationship which, in the opinion of our Board, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Rule 5605(a)(2) generally provides that a director cannot be considered independent if:
| • | the director is, or at any time during the past three years was, an employee of the company; |
| • | the director or a family member of the director accepted any compensation from the company in excess of $120,000 during any period of 12 consecutive months within the three years preceding the independence determination (subject to certain exemptions, including, among other things, compensation for board or board committee service); |
| • | the director is an immediate family member of an individual who is, or at any time during the past three years was, employed by the company as an executive officer; |
| • | the director or a family member of the director is a partner in, controlling stockholder of, or an executive officer of an entity to which the company made, or from which the company received, payments in the current or any of the past three fiscal years that exceed 5% of the recipient’s consolidated gross revenue for that year or $200,000, whichever is greater (subject to certain exemptions); |
| • | the director or a family member of the director is employed as an executive officer of an entity where, at any time during the past three years, any of the executive officers of the company served on the compensation committee of such other entity; or |
| • | the director or a family member of the director is a current partner of the company’s outside auditor, or at any time during the past three years was a partner or employee of the company’s outside auditor, and who worked on the company’s audit. |
Consistent with these considerations, after review of all relevant identified transactions or relationships between each director, or any of his or her family members, and us, our senior management and our independent auditors, the Board has affirmatively determined that the following four directors are independent directors as defined by Rule 5605(a)(2) of the Nasdaq Listing Rules: Mr. Gustafson, Ms. McGrath, Dr. Woo and Mr. Oram. In making this determination, the Board found that none of these directors had a material or other disqualifying relationship with us. Messrs. Jackman, Nisser and Horne are not considered independent because of either their current employment with us or their relationship with our significant shareholders.
Board Committees
Our Board of Directors has an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee. The responsibilities of the Audit Committee (which consists of Mr. Gustafson (Chair), Mr. Oram and Dr. Woo) include recommending to the Board of Directors the firm of independent accountants to be retained by our company, reviewing with our independent accountants the scope and results of their audits, and reviewing with the independent accountants and management our accounting and reporting principles, policies and practices, as well as our accounting, financial and operating controls and staff. The Compensation Committee (which consist of Mr. Oram (Chair), Mr. Gustafson and Dr. McGrath) has responsibility for establishing and reviewing employee compensation. The Compensation Committee also has responsibility for administering and interpreting the Alzamend Neuro, Inc. 2021 Stock Incentive Plan, and determining the recipients, amounts and other terms (subject to the requirements of the Plan) of stock options and other equity-based awards which may be granted under the 2021 Stock Incentive Plan from time to time. The purpose of the Nominating and Corporate Governance Committee (which consist of Dr. McGrath (Chair) and Dr. Woo) is to select, or recommend for our entire Board’s selection, the individuals to stand for election as directors at the annual meeting of stockholders, as well as to consider the adequacy of our corporate governance and oversee and approve management continuity planning processes.
| - 66 - |
Certain Board Arrangements
In May 2021, the Board of Directors of our company and Mr. Ault, our Founder and Chairman Emeritus, agreed to certain arrangements with regard to our Board composition and other matters. Contemporaneously with the effectiveness of the initial public offering, and in consideration for (i) the conversion of 750 shares of our series A convertible preferred stock beneficially owned by Mr. Ault through ALSI into 15,000,000 shares of our common stock, (ii) the extension of the maturity date of the note in the original principal amount of $15,000,000 issued to us by ALSF to December 31, 2023, and (iii) the retirement by Mr. Ault as a director and executive officer of our company, the Board agreed that William B. Horne will become our Chairman of the Board and remain in that position for so long as Mr. Ault beneficially owns no less than 5% of the outstanding shares of our common stock (for which Mr. Horne will be paid $50,000 per year for his services), and Mr. Nisser will remain a member of our Board of Directors for so long as Mr. Ault beneficially owns no less than 5% of the outstanding shares of our common stock (for no additional remuneration). Additionally, Mr. Ault will hold the position of Founder and Chairman Emeritus and, as such, have the right to nominate an observer to our Board of Directors for a period of five years after the closing date of the initial public offering. Following the closing of the initial public offering, we entered into a five-year consulting agreement with Mr. Ault under which he will provide strategic advisory and consulting services to us in consideration for annual fees of $50,000.
| ITEM 11. | EXECUTIVE COMPENSATION |
Summary Compensation Table
The following table sets forth summary compensation information for the following persons: (i) all persons serving as our principal executive officer during the years ended April 30, 2022 and 2021, and (ii) our two other most highly compensated executive officers who received compensation during the years ended April 30, 2022 and 2021, who were executive officers on the last day of our fiscal year. We refer to these persons as our “named executive officers” in this Annual Report. The following table includes all compensation earned by the named executive officers for the respective period, regardless of whether such amounts were actually paid during the period:
| Name and principal position | Year | Salary ($) | Bonus ($) |
Stock award ($) |
Option Awards⁽¹⁾ ($) |
All Other Compensation ($) |
Total ($) | |||||||||||||||||||||
| Stephan S. Jackman | 2022 | 303,125 | 170,000 | — | — | — | 473,125 | |||||||||||||||||||||
| Chief Executive Officer | 2021 | 225,000 | — | — | — | — | 225,000 | |||||||||||||||||||||
| Lien Escalona | 2022 | 105,000 | — | — | 1,077,302 | — | 1,182,302 | |||||||||||||||||||||
| Chief Financial Officer | ||||||||||||||||||||||||||||
| Kenneth S. Cragun | 2021 | 100,000 | — | — | — | — | 100,000 | |||||||||||||||||||||
| Senior VP of Finance | ||||||||||||||||||||||||||||
| (1) | The values reported in the “Option Awards” column represents the aggregate grant date fair value, computed in accordance with Accounting Standards Codification (“ASC”) 718 Share Based Payments, of grants of stock options to each of our named executive officers and directors. |
| - 67 - |
Employment Agreements
Stephan Jackman. On June 17, 2021, we entered into an employment agreement (the “Agreement”) with Stephan Jackman to continue to serve as our Chief Executive Officer through July 1, 2024. Pursuant to the Agreement, Mr. Jackman will be paid a base salary of $300,000 per annum (the “Base Salary”). In addition, Mr. Jackman shall be eligible to earn a cash and/or equity bonus as our Board of Directors (the “Board”) may determine, from time to time, based on meeting performance objectives and bonus criteria to be identified by the Board (the “Performance Bonus”), which Performance Bonus may consist of cash or, in the Board’s sole discretion, our common stock. The determination of whether we have achieved a certain financial performance objective in any year for the purposes of the Performance Bonus shall be made by our independent registered public accounting firm regularly retained or employed by us within 90 days after the end of each fiscal year.
Further, Mr. Jackman is entitled to receive equity participation as follows: (A) options to purchase 5,000,000 shares of common stock, which options were previously granted and are exercisable for a period of 10 years at an exercise price of $1.00 per share (the “$1.00 Options”), and (B) options to purchase 2,000,000 shares of our common stock, which options shall be exercisable for a period of 10 years at an exercise price of $1.50 per share (the “$1.50 Options”, and collectively with the $1.00 Options, the “Options”).
Subject to the terms and conditions set forth in the Agreement, the Options shall vest pursuant to the following schedule: (1) 3,000,000 shares of common stock subject to the $1.00 Options shall vest ratably over 48 months, commencing on November 16, 2018; (2) 1,000,000 shares of common stock subject to the $1.00 Options shall vest upon approval of a NDA for AL001 by the FDA, provided that such approval occurs on or prior to November 1, 2022; (3) 1,000,000 shares of common stock subject to the $1.00 Options shall vest upon the approval of an NDA for AL002 by the FDA, provided that such approval occurs on or prior to November 1, 2022; and (4) the $1.50 Options shall vest upon satisfaction of mutually agreed upon performance criteria as set forth in Mr. Jackman’s Non-Qualified Stock Option Grant dated November 26, 2019.
Mr. Jackman’s bonuses, if any, and all stock based compensation shall be subject to “Company Clawback Rights” if during the period that Mr. Jackman is employed by us and upon the termination of Mr. Jackman’s employment and for a period of two years thereafter, if there is a restatement of any of our financial results from which any bonuses and stock based compensation to Mr. Jackman shall have been determined.
Upon termination of Mr. Jackman’s employment (other than upon the expiration of the employment), Mr. Jackman shall be entitled to receive: (A) any earned but unpaid Base Salary through the termination date; (B) all reasonable expenses paid or incurred; and (C) any accrued but unused vacation time.
Further, unless Mr. Jackman’s employment is terminated as a result of his death or disability or for cause or he terminates his employment without good reason, then upon the termination of Mr. Jackman’s employment, the Company shall pay to Mr. Jackman a “Separation Payment” as follows: (a) an amount equal to 12 months of the Base Salary (as in effect immediately prior to the termination date); and (b) a prorated Performance Bonus amount calculated in accordance with the Performance Bonus criteria set forth in the Agreement and the actual number of days Mr. Jackman worked in the calendar year prior to the termination date. In addition, all of Mr. Jackman’s Options shall immediately vest and shall be exercisable for a period of 12 months after such termination.
Kenneth S. Cragun. In November 2018, we entered into an offer letter with Kenneth S. Cragun to serve as our Chief Financial Officer for a period of four years. For his services, Mr. Cragun is paid a base salary of $100,000 per year, which amount would be increased to $120,000 upon the approval of a listing application submitted on behalf of our company to have our shares of common stock listed on a national securities exchange. In addition, Mr. Cragun will be eligible to receive an annual cash bonus equal to a percentage of his annual base salary based on achievement of applicable performance goals determined by the Board. The annual bonus, if any, will in part be determined based upon the successful attainment of the same milestones as are applicable for Mr. Jackman. In June 2021, Mr. Cragun became our Senior Vice President of Finance.
Mr. Cragun received a stock option to purchase 1,500,000 shares of our common stock exercisable for a period of 10 years from December 15, 2018 at a per share price of $1.00. The option will vest in equal increments over 48 months beginning on December 15, 2018; however, 500,000 shares of our common stock vested immediately upon the approval of a listing application submitted on behalf of our company to have our shares of common stock listed on a national securities exchange.
In November 2019, the Board of Directors granted 1,000,000 performance- and market-contingent awards to Mr. Cragun. These awards have an exercise price of $1.50 per share. These awards have multiple separate market triggers for vesting based upon either (i) the successful achievement of stepped target closing prices on a national securities exchange for 90 consecutive trading days later than 180 days after our initial public offering of common stock, or (ii) stepped target prices for a change in control transaction. The target prices range from $15 per share to $40 per share. In the event any the stock price milestones are not achieved within three years, the unvested portion of the performance options will be reduced by 25%.
| - 68 - |
Henry Nisser. In May 2019, we entered into a four-year employment agreement with Henry C.W. Nisser to serve as our Executive Vice President and General Counsel. For his services, Mr. Nisser is paid a base salary of $50,000 per year and is eligible to receive an annual cash bonus equal to a percentage of his annual base salary based on achievement of applicable performance goals determined by our Board of Directors.
Mr. Nisser received a stock option to purchase 1,250,000 shares of our common stock exercisable for a period of five years at an exercise price of $1.50 per share. The shares of our common stock underlying the option vest in equal monthly installments over the 48 months beginning on June 1, 2019.
Outstanding Equity Awards at Fiscal Year End
The following table provides information on outstanding equity awards as of April 30, 2022 awarded to our named executive officers:
| OUTSTANDING EQUITY AWARDS AT APRIL 30, 2022 | ||||||||||||||||||||
| Option Awards | ||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | |||||||||||||||
| Stephan Jackman | - | 1,000,000 | 1,000,000 | 1.00 | 11/01/2022 | |||||||||||||||
| - | 1,000,000 | 1,000,000 | 1.00 | 11/01/2022 | ||||||||||||||||
| 2,562,500 | 437,500 | - | 1.00 | 11/15/2028 | ||||||||||||||||
| - | 2,000,000 | 2,000,000 | 1.50 | 11/18/2029 | ||||||||||||||||
| Lien T. Escalona | 83,330 | 216,670 | - | 5.00 | 8/13/2031 | |||||||||||||||
| Kenneth S. Cragun | 1,250,000 | 250,000 | - | 1.00 | 12/15/2028 | |||||||||||||||
| - | 1,000,000 | 1,000,000 | 1.50 | 11/18/2029 | ||||||||||||||||
Incentive Compensation Plans
2016 Stock Incentive Plan
In April 2016, our stockholders approved our company’s 2016 Stock Incentive Plan (the “2016 Plan”). The 2016 Plan provides for the issuance of a maximum of 12,500,000 shares of our common stock to be offered to our directors, officers, employees and consultants. On March 1, 2019, our stockholders approved an additional 7,500,000 shares to be available for issuance under the 2016 Plan. Options granted under the 2016 Plan have an exercise price equal to or greater than the fair value of the underlying common stock at the date of grant and become exercisable based on a vesting schedule determined at the date of grant. The options expire between five and 10 years from the date of grant. Restricted stock awards granted under the 2016 Plan are subject to a vesting period determined at the date of grant.
2021 Stock Incentive Plan
In February 2021, our Board of Directors adopted, and our stockholders approved, the Alzamend Neuro, Inc. 2021 Stock Incentive Plan (the “2021 Plan”). The 2021 Plan authorizes the grant to eligible individuals of (1) stock options (incentive and non-statutory), (2) restricted stock, (3) stock appreciation rights, or SARs, (4) restricted stock units, and (5) other stock-based compensation.
Stock Subject to the 2021 Plan. The maximum number of shares of our common stock that may be issued under the 2021 Plan is 10,000,000 shares, which number will be increased to the extent that compensation granted under the 2021 Plan is forfeited, expires or is settled for cash (except as otherwise provided in the 2021 Plan). Substitute awards (awards made or shares issued by us in assumption of, or in substitution or exchange for, awards previously granted, or the right or obligation to make future awards, in each case by a company that we acquire or any subsidiary of ours or with which we or any subsidiary combines) will not reduce the shares authorized for grant under the 2021 Plan, nor will shares subject to a substitute award be added to the shares available for issuance or transfer under the 2021 Plan.
| - 69 - |
No Liberal Share Recycling. Notwithstanding anything to the contrary, any and all stock that is (i) withheld or tendered in payment of an option exercise price; (ii) withheld by us or tendered by the grantee to satisfy any tax withholding obligation with respect to any award; (iii) covered by a SAR that it is settled in stock, without regard to the number of shares of stock that are actually issued to the grantee upon exercise; or (iv) reacquired by us on the open market or otherwise using cash proceeds from the exercise of options, will not be added to the maximum number of shares of stock that may be issued under the 2021 Plan.
Eligibility. Employees of, and consultants to, our company or our affiliates and members of our Board of Directors are eligible to receive equity awards under the 2021 Plan. Only our employees, and employees of our parent and subsidiary corporations, if any, are eligible to receive incentive stock options. Employees, directors (including non-employee directors) and consultants of or for our company and our affiliates are eligible to receive non-statutory stock options, restricted stock, purchase rights and any other form of award the 2021 Plan authorizes.
Purpose. The purpose of the 2021 Plan is to promote the interests of our company and our stockholders by providing executive officers, employees, non-employee directors, and key advisors of our company and our subsidiaries with appropriate incentives and rewards to encourage them to enter into and remain in their positions with us and to acquire a proprietary interest in our long-term success, as well as to reward the performance of these individuals in fulfilling their personal responsibilities for long-range and annual achievements.
Administration. Unless otherwise determined by the Board of Directors, the Compensation Committee administers the 2021 Plan. The Compensation Committee is composed solely of “non-employee directors” within the meaning of Rule 16b-3 under the Exchange Act, “outside directors” within the meaning of Section 162(m) of the Internal Revenue Code, and “independent directors” within the meaning of the Nasdaq Marketplace Rules. The Compensation Committee has the power, in its discretion, to grant awards under the 2021 Plan, to select the individuals to whom awards are granted, to determine the terms of the grants, to interpret the provisions of the 2021 Plan and to otherwise administer the 2021 Plan. Except as prohibited by applicable law or any rule promulgated by a national securities exchange to which our company may in the future be subject, the Compensation Committee may delegate all or any of its responsibilities and powers under the 2021 Plan to one or more of its members, including, without limitation, the power to designate participants and determine the amount, timing and term of awards under the 2021 Plan. In no event, however, will the Compensation Committee have the power to accelerate the payment or vesting of any award, other than in the event of death, disability, retirement or a change of control of our company.
The 2021 Plan provides that members of the Compensation Committee will be indemnified and held harmless by us from any loss or expense resulting from claims and litigation arising from actions related to the 2021 Plan.
Term. The 2021 Plan was effective as of February 17, 2021, and awards may be granted through February 16, 2031. No awards may be granted under the 2021 Plan subsequent to that date. The Board of Directors may suspend or terminate the 2021 Plan without stockholder approval or ratification at any time or from time to time.
Amendments. Subject to the terms of the 2021 Plan, the Compensation Committee, as administrator, has the sole discretion to interpret the provisions of the 2021 Plan and outstanding awards. Our Board of Directors generally may amend or terminate the 2021 Plan at any time and for any reason, except that no amendment, suspension or termination may impair the rights of any participant without his or her consent, and except that approval of our stockholders is required for any amendment which, among provisions, increases the number of shares of common stock subject to the 2021 Plan, decreases the price at which grants may be granted and reprices existing options.
Repricing Prohibition. Other than in connection with certain corporate events, the Compensation Committee will not, without the approval of our stockholders, (a) lower the option price per share of an option or SAR after it is granted, (b) cancel an option or SAR when the exercise price per share exceeds the fair market value of one share in exchange for cash or another award (other than in connection with a change of control), or (c) take any other action with respect to an option or SAR that would be treated as a repricing under the rules and regulations of the principal U.S. national securities exchange on which our shares are then listed.
Minimum Vesting Requirement. Grantees of full-value awards (i.e., awards other than options and SARs), will be required to continue to provide services to us or an affiliated company) for not less than one-year following the date of grant in order for any such full-value awards to fully or partially vest (other than in case of death, disability or a Change of Control). Notwithstanding the foregoing, up to 5% of the available shares of stock authorized for issuance under the 2021 Plan may provide for vesting of full-value awards, partially or in full, in less than one year.
| - 70 - |
Adjustments upon Changes in Capitalization. In the event of any merger, reorganization, consolidation, recapitalization, dividend or distribution (whether in cash, shares or other property, other than a regular cash dividend), stock split, reverse stock split, spin-off or similar transaction or other change in our corporate structure affecting our common stock or the value thereof, appropriate adjustments to the 2021 Plan and awards will be made as the Board of Directors determines to be equitable or appropriate, including adjustments in the number and class of shares of stock available for issuance under the 2021 Plan, the number, class and exercise or grant price of shares subject to awards outstanding under the 2021 Plan, and the limits on the number of awards that any person may receive.
Change of Control. Agreements evidencing awards under the 2021 Plan may provide that upon a Change of Control (as defined in the 2021 Plan), unless otherwise provided in the agreement evidencing an award), outstanding awards may be cancelled and terminated without payment if the consideration payable with respect to one share of stock in connection with the Change of Control is less than the exercise price or grant price applicable to such award, as applicable.
Notwithstanding any other provisions of the 2021 Plan to the contrary, the vesting, payment, purchase or distribution of an award may not be accelerated by reason of a Change of Control for any participant unless the Grantee’s employment is involuntarily terminated as a result of the Change of Control as provided in the Award agreement or in any other written agreement, including an employment agreement, between us and the participant. If the Change of Control results in the involuntary termination of participant’s employment, outstanding awards will immediately vest, become fully exercisable and may thereafter be exercised.
Generally, under the 2021 Plan, a Change of Control occurs upon (i) the consummation of a reorganization, merger or consolidation of our company with or into another entity, pursuant to which our stockholders immediately prior to the transaction do not own more than 50% of the total combined voting power after the transaction, (ii) the consummation of the sale, transfer or other disposition of all or substantially all of our assets, (iii) certain changes in the majority of our Board of Directors from those in office on the effective date of the 2021 Plan, (iv) the acquisition of more than 50% of the total combined voting power in our outstanding securities by any person, or (v) we are dissolved or liquidated.
Types of Awards
Stock Options. Incentive stock options and non-statutory stock options are granted pursuant to award agreements adopted by our Compensation Committee. Our Compensation Committee determines the exercise price for a stock option, within the terms and conditions of the 2021 Plan; provided, that the exercise price of an incentive stock option cannot be less than 100% of the fair market value of our common stock on the date of grant. Options granted under the 2021 Plan vest at the rate specified by our Compensation Committee.
The Compensation Committee determines the term of stock options granted under the 2021 Plan, up to a maximum of 10 years, except in the case of certain Incentive Stock Options, as described below. The Compensation Committee will also determine the length of period during which an optionee may exercise their options if an optionee’s relationship with us, or any of our affiliates, ceases for any reason; for incentive stock options, this period is limited by applicable law. The Compensation Committee may extend the exercise period in the event that exercise of the option following termination of service is prohibited by applicable securities laws. In no event, however, may an option be exercised beyond the expiration of its term unless the term is extended in accordance with applicable law.
Acceptable consideration for the purchase of common stock issued upon the exercise of a stock option will be determined by the Compensation Committee and may include (a) cash or its equivalent, (b) delivering a properly executed notice of exercise of the option to us and a broker, with irrevocable instructions to the broker promptly to deliver to us the amount necessary to pay the exercise price of the option, (c) any other form of legal consideration that may be acceptable to the Compensation Committee or (d) any combination of (a), (b) or (c).
Unless the Compensation Committee provides otherwise, options are generally transferable in accordance with applicable law, provided that any transferee of such options agrees to become bound by the terms of the 2021 Plan. An optionee may also designate a beneficiary who may exercise the option following the optionee’s death.
Incentive or Non-statutory Stock Options. Incentive stock options may be granted only to our employees, and the employees of our parent or subsidiary corporations, if any. The Compensation Committee may grant awards of incentive or non-statutory stock options that are fully vested on the date made, to any of our employees, directors or consultants. Option awards are granted pursuant to award agreements adopted by our Compensation Committee. To the extent required by applicable law, the aggregate fair market value, determined at the time of grant, of shares of our common stock with respect to incentive stock options that are exercisable for the first time by an optionee during any calendar year may not exceed $100,000. To the extent required by applicable law, no incentive stock option may be granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power or that of any of our affiliates unless (a) the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant and (b) the term of the incentive stock option does not exceed five years from the date of grant.
| - 71 - |
Stock Appreciation Rights. An SAR is the right to receive stock, cash, or other property equal in value to the difference between the grant price of the SAR and the market price of our common stock on the exercise date. SARs may be granted independently or in tandem with an option at the time of grant of the related option. An SAR granted in tandem with an option will be exercisable only to the extent the underlying option is exercisable. An SAR confers on the grantee a right to receive an amount with respect to each share of common stock subject thereto, upon exercise thereof, equal to the excess of (A) the fair market value of one share of common stock on the date of exercise over (B) the grant price of the SAR (which in the case of an SAR granted in tandem with an option will be equal to the exercise price of the underlying option, and which in the case of any other SAR will be such price as the Compensation Committee may determine but in no event will be less than the fair market value of a share of common stock on the date of grant of such SAR).
Restricted Stock and Restricted Stock Units. Restricted stock is common stock that we grant subject to transfer restrictions and vesting criteria. A restricted stock unit is a right to receive stock or cash equal to the value of a share of stock at the end of a specified period that we grant subject to transfer restrictions and vesting criteria. The grant of these awards under the 2021 Plan are subject to such terms, conditions and restrictions as the Compensation Committee determines consistent with the terms of the 2021 Plan.
At the time of grant, the Compensation Committee may place restrictions on restricted stock and restricted stock units that will lapse, in whole or in part, only upon the attainment of performance goals; provided that such performance goals will relate to periods of performance of at least one fiscal year, and if the award is granted to a 162(m) officer, the grant of the award and the establishment of the performance goals will be made during the period required under Internal Revenue Code Section 162(m). Except to the extent restricted under the award agreement relating to the restricted stock, a grantee granted restricted stock will have all of the rights of a stockholder, including the right to vote restricted stock and the right to receive dividends.
Unless otherwise provided in an award agreement, upon the vesting of a restricted stock unit, there will be delivered to the grantee, within 30 days of the date on which such award (or any portion thereof) vests, the number of shares of common stock equal to the number of restricted stock units becoming so vested.
Other Stock-Based Awards. The 2021 Plan also allows the Compensation Committee to grant “Other Stock-Based Awards,” which means a right or other interest that may be denominated or payable in, valued in whole or in part by reference to, or otherwise based on, or related to, common stock. Subject to the limitations contained in the 2021 Plan, this includes, without limitation, (i) unrestricted stock awarded as a bonus or upon the attainment of performance goals or otherwise as permitted under the 2021 Plan, and (ii) a right to acquire stock from us containing terms and conditions prescribed by the Compensation Committee. At the time of the grant of other stock-based awards, the Compensation Committee may place restrictions on the payout or vesting of other stock-based awards that will lapse, in whole or in part, only upon the attainment of performance goals; provided that such Performance Goals will relate to periods of performance of at least one fiscal year, and if the award is granted to a 162(m) Officer, the grant of the Award and the establishment of the performance goals will be made during the period required under Internal Revenue Code Section 162(m). Other Stock-Based Awards may not be granted with the right to receive dividend equivalent payments.
Performance Awards. Performance awards provide participants with the opportunity to receive shares of our common stock, cash or other property based on performance and other vesting conditions. Performance awards may be granted from time to time as determined at the discretion of the Board, or the Compensation Committee (as applicable). Subject to the share limit and maximum dollar value set forth above under “Limits per Participant,” the Board, or the Compensation Committee (as applicable), has the discretion to determine (i) the number of shares of common stock under, or the dollar value of, a performance award and (ii) the conditions that must be satisfied for grant or for vesting, which typically will be based principally or solely on achievement of performance goals.
Performance Criteria. With respect to awards intended to qualify as performance-based compensation under Code Section 162(m), a committee of “outside directors” (as defined in Code Section 162(m)) with authority delegated by our Board will determine the terms and conditions of such awards, including the performance criteria. The performance goals for restricted stock awards, restricted stock units, performance awards or other share-based awards will be based on the attainment of specified levels of, among other metrics, the attainment of certain target levels of, or a specified percentage increase in, revenues, earnings, income before taxes and extraordinary items, net income, operating income, earnings before or after deduction for all or any portion of income tax, earnings before interest, taxes, depreciation and amortization or a combination of any or all of the foregoing.
The performance goals may be based solely by reference to our performance or the performance of one or more of our subsidiaries, parents, divisions, business segments or business units, or based upon the relative performance of other companies or upon comparisons of any of the indicators of performance relative to other companies. The authorized committee of outside directors may also exclude under the terms of the performance awards, the impact of an event or occurrence that the committee determines should appropriately be excluded, including restructurings, discontinued operations, extraordinary items, and other unusual or non-recurring charges, or changes in generally accepted accounting principles or practices.
| - 72 - |
Director Compensation
The Company pays each independent director an annual base amount of $25,000. In April 2022, the Board approved a bonus payment of $50,000 for each independent director. Additionally, our Board makes recommendations for adjustments to an independent director’s compensation when the level of services provided are significantly above what was anticipated.
The table below sets forth, for each non-employee director, the total amount of compensation related to his or her service during the year ended April 30, 2022:
| Name | Fees earned or paid in cash ($) | Stock awards ($) | Options awards ($) | All other compensation ($) | Total ($) | |||||||||||||||
| William B. Horne | 43,756 | — | — | — | 43,756 | |||||||||||||||
| Mark Gustafson | 64,583 | 250,000 | 530,897 | — | 845,480 | |||||||||||||||
| Lynne Fahey McGrath | 64,583 | 250,000 | 530,897 | — | 845,480 | |||||||||||||||
| Andy H. Woo | 64,583 | 250,000 | 530,897 | — | 845,480 | |||||||||||||||
| Jeffrey Oram | 64,583 | 250,000 | 530,897 | — | 845,480 | |||||||||||||||
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The following table shows the beneficial ownership of our common stock as of July 19, 2022, held by (i) each person known by us to be the beneficial owner of more than 5% of our outstanding common stock, (ii) each of our directors and director nominees, (iii) each of our executive officers, and (iv) all of our directors, director nominees and executive officers as a group. As of the date of this Annual Report, there were 95,481,790 shares of our common stock issued and outstanding.
Beneficial ownership is determined in accordance with the rules of the SEC, and generally includes voting power and/or investment power with respect to the securities held. Shares of our common stock subject to options and warrants currently exercisable or which may become exercisable within 60 days of the date of this Annual Report, are deemed outstanding and beneficially owned by the person holding such options or warrants for purposes of computing the number of shares and percentage beneficially owned by such person but are not deemed outstanding for purposes of computing the percentage beneficially owned by any other person. Except as indicated in the footnotes to this table, the persons or entities named have sole voting and investment power with respect to all shares of our common stock shown as beneficially owned by them.
Unless otherwise noted in the footnotes to the following table, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to their beneficially owned common stock.
Unless otherwise indicated, the principal address of each of the persons below is c/o Alzamend Neuro, Inc., 3500 Lenox Rd NE, Suite 1500, Atlanta, GA 30326.
| - 73 - |
| Greater than 5% Beneficial Owners: | Number of shares of Common Stock Beneficially Owned | Percentage of Shares Beneficially Owned | ||||||
| Milton C. Ault, III (1) (2) (3) (4) | 42,718,318 | 42.51 | % | |||||
| Ault Life Sciences, Inc. (1) | 14,942,984 | 15.65 | % | |||||
| Ault Life Sciences Fund, LLC (2) | 15,000,000 | 14.93 | % | |||||
| Digital Power Lending, LLC (3) | 9,933,667 | 10.40 | % | |||||
| Directors and Executive Officers | ||||||||
| Stephan Jackman (5) | 2,875,000 | 2.92 | % | |||||
| Henry C.W. Nisser (5) | 1,041,667 | 1.08 | % | |||||
| Kenneth S. Cragun (5) | 1,406,250 | 1.45 | % | |||||
| David J. Katzoff (6) | 1,159,292 | 1.20 | % | |||||
| Lien T. Escalona (5) | 100,000 | * | ||||||
| William B. Horne (7) | 2,729,167 | 2.79 | % | |||||
| Mark Gustafson (8) | 210,000 | * | ||||||
| Lynne Fahey McGrath, M.P.H., Ph.D. (9) | 225,000 | * | ||||||
| Jeffrey Oram (10) | 250,000 | * | ||||||
| Andrew H. Woo, M.D., Ph.D. (10) | 250,000 | * | ||||||
| All directors and named executive officers as a group (10 persons) | 10,246,375 | 9.77 | % | |||||
* Less than 1% of outstanding shares.
| (1) | Milton C. (Todd) Ault III, our Founder and Chairman Emeritus, has sole voting and investment power with respect to the shares held of record by ALSI. |
| (2) | Represents 10,000,000 shares of our common stock and 5,000,000 shares of our common stock issuable upon the exercise of warrants. Mr. Ault has sole voting and investment power with respect to the securities held of record by ALSF. |
| (3) | Represents 9,926,667 shares of our common stock held by DPL and 7,000 shares of our common stock purchasable upon the exercise of call options (right to buy). Mr. Ault has voting and investment power with respect to the securities held by DPL. Excludes 3,333,333 shares of our common stock underlying currently exercisable warrants held by DPL due to a beneficial ownership blocker limitation provision contained therein. |
| (4) | Includes (i) 2,500,000 shares of our common stock held by Mr. Ault, (ii) 325,000 shares of our common stock held by Ault Alpha LP, and (iii) 16,667 shares of common stock issuable upon the exercise of warrants held by BitNile Holdings, Inc. Mr. Ault is the Manager of Ault Alpha GP LLC ("Ault GP") and Ault Capital Management LLC ("AC Management"). Ault GP and AC Management are the general partner and investment manager to Ault Alpha LP, respectively. As such, Mr. Ault is deemed to beneficially own the shares held by Ault Alpha LP. |
| (5) | Represents shares of our common stock issuable upon the exercise of stock options, which are currently exercisable or exercisable within 60 days. Mr. Nisser’s address is 100 Park Avenue, Suite 1658, New York, New York 10017. |
| (6) | Consists of 18,000 shares of our common stock, 9,000 shares of our common stock issuable upon the exercise of warrants and 1,132,292 shares of our common stock issuable upon the exercise of stock options that are currently exercisable or exercisable within 60 days. |
| (7) | Consists of 500,000 shares of our common stock and 2,229,167 shares of our common stock issuable upon the exercise of stock options that are currently exercisable or exercisable within 60 days. |
| (8) | Consists of 60,000 shares of our common stock and 150,000 shares of our common stock issuable upon the exercise of stock options that are currently exercisable or exercisable within 60. |
| (9) | Consists of 75,000 shares of our common stock and 150,000 shares of our common stock issuable upon the exercise of stock options that are currently exercisable or exercisable within 60. |
| (10) | Consists of 100,000 shares of our common stock and 150,000 shares of our common stock issuable upon the exercise of stock options that are currently exercisable or exercisable within 60. |
| - 74 - |
Equity Compensation Information
The following table summarizes information about our equity compensation plans as of April 30, 2022.
| Number of securities | ||||||||||||
| Number of securities | Weighted- | remaining available for | ||||||||||
| to be issued | average | future issuance under | ||||||||||
| upon exercise | exercise price | equity compensation plans | ||||||||||
| of outstanding | of outstanding | (excluding securities | ||||||||||
| options, warrants and rights | options, warrants and rights | reflected in column (a)) | ||||||||||
| Plan Category | (a) | (b) | (c) | |||||||||
| Equity compensation plans approved by stockholders | 15,700,000 | 1.20 | 8,800,000 | |||||||||
| Equity compensation plans not approved by stockholders | - | - | - | |||||||||
| Total | 15,700,000 | 1.20 | 8,800,000 | |||||||||
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS AND DIRECTOR INDEPENDENCE |
Certain Relationships
Our company is controlled by Milton C. (Todd) Ault III, our Founder and current Chairman Emeritus, directly and through his controlling interests in DPL, ALSI and ALSF. Mr. Ault is also the Chairman, Chief Executive Officer and single largest stockholder (through Ault Alpha LP) of BitNile. The Board of Directors and executive officers of our company and the board of directors and executive officers of BitNile contain some of the same individuals. William B. Horne, the Chairman of the Board of our company, is the Chief Executive Officer and a director of BitNile, Henry C.W. Nisser, our Executive Vice President, General Counsel and a director of our company, is the President, General Counsel and a director of BitNile, and Kenneth S. Cragun, our Senior Vice President of Finance is the Chief Financial Officer of BitNile. Additionally, Mr. Ault is the Chairman of Avalanche, of which Mr. Horne is a director and its Chief Financial Officer and Mr. Nisser is its Executive Vice President and General Counsel.
Transactions with Related Persons
To the best of our knowledge, during our most recent fiscal year end on April 30, 2022, other than as set forth below, there were no material transactions, or series of similar transactions, or any currently proposed transactions, or series of similar transactions, to which we were or are to be a party, in which the amount involved exceeds $87,145, or 1% of the average total assets at year-end for the last two completed fiscal years, and in which any director or executive officer, or any security holder who is known by us to own of record or beneficially more than 5% of any class of our common stock, or any member of the immediate family of any of the foregoing persons, has an interest (other than compensation to our officers and directors in the ordinary course of business).
On April 10, 2018, we entered into a note receivable agreement with Avalanche in the amount of $995,500, subject to the terms and conditions stated in the AVLP Note. The AVLP Note accrued interest at 10% per annum and included a 10% original issue discount. The balance outstanding on the AVLP Note as of April 30, 2020 was $100,915. In August 2020, the principal and accrued interest on the AVLP Note was paid in full.
On April 30, 2019, we entered into a securities purchase agreement with ALSF for the sale of 10,000,000 shares of our common stock, plus 5,000,000 warrants with a five-year term and an exercise price of $3.00 per share and vesting upon issuance (the “ALSF Warrants”). The total purchase price of $15,000,000 was in the form of a note from ALSF. The note balance as of April 30, 2020 was reduced by $16,800 reflecting payments made during the year ended April 30, 2020. The note balance as of April 30, 2021 was reduced by $99,905 reflecting payments made during the year ended April 30, 2021. As of April 30, 2022, the note balance was $14,883,295. The control person of ALSF is Mr. Ault, our Founder and Chairman Emeritus. ALSF is wholly owned by ALSI. ALSI is almost entirely wholly owned by Ault & Co., Inc., of which MCKEA Holdings, LLC (“MCKEA”), of which Mr. Ault’s spouse is the managing member, is the majority owner. As such, MCKEA is indirectly the majority owner of ALSF.
The note is secured by a Stock Pledge Agreement dated June 11, 2019. While the securities purchase agreement provides for ALSF’s ability to pledge the securities acquired thereby, given that the purchased securities are subject to the securities purchase agreement, we and ALSF agreed that such securities may not be pledged to any third party until the current pledge agreement has been terminated through full repayment of the note.
| - 75 - |
Pursuant to the securities purchase agreement, ALSF is entitled to full ratchet anti-dilution protection, most-favored nation status, denying our company the right to enter into a variable rate transaction absent its consent, and the right to participate in any future financing we may consummate. All these rights, other than the right to participate in future financings which will not terminate until ALSF no longer holds any shares of our common stock or any ALSF Warrants, will terminate on the earlier to occur of such date that we have (i) completed a Qualified Financing, or (ii) received approval by the FDA for any of our product candidates in Phase III clinical trial. For purposes of the securities purchase agreement, a “Qualified Financing” means the sale of equity securities by us in a single transaction or a series of related transactions whether or not registered under the Securities Act, resulting in gross proceeds to us of no less than $25,000,000.
In March 2021, we entered into a securities purchase agreement with Digital Power Lending, LLC (“DPL”), a California limited liability company and wholly-owned subsidiary of BitNile, pursuant to which we agreed to sell 6,666,667 shares of our common stock for an aggregate of $10 million, or $1.50 per share, which sales will be made in tranches. On March 9, 2021, DPL paid $4 million, less the $1.8 million in advances and the surrender for cancellation of a $50,000 convertible promissory note for 2,666,667 shares of our common stock. Under the terms of the securities purchase agreement, DPL purchased an additional (i) 1,333,333 shares of our common stock upon approval by the FDA of our IND for our opening Phase I clinical trial for a purchase price of $2 million, and (ii) 2,666,667 shares of our common stock once we completed the opening Phase I clinical trial for a purchase price of $4 million. We met the first milestone on July 28, 2021 and the second milestone in the fourth fiscal quarter of 2022. In addition, we issued DPL warrants to purchase an aggregate of 6,666,667 shares of common stock at an exercise price of $3.00 per share. Finally, we agreed that for a period of 18 months following the date of the payment of the final tranche of $4 million, DPL will have the right to invest an additional $10 million on the same terms, except that no specific milestones have been determined with respect to the additional $10 million investment as of the date of this Annual Report.
In May 2021, the Board of Directors of our company and Mr. Ault, our Founder and Chairman Emeritus, agreed to certain arrangements with regard to our Board composition and other matters. Contemporaneously with the consummation of the initial public offering, and in consideration for (i) the conversion of 750 shares of our series A convertible preferred stock beneficially owned by Mr. Ault through ALSI into 15,000,000 shares of our common stock, (ii) the extension of the maturity date of the note in the original principal amount of $15,000,000 issued to us by ALSF to December 31, 2023, and (iii) the resignation of Mr. Ault as a director and executive officer of our company, the Board agreed that William B. Horne be named our Chairman of the Board and remain in that position for so long as Mr. Ault beneficially owns no less than 5% of the outstanding shares of our common stock (for which Mr. Horne will be paid $50,000 per year for his services), and Mr. Nisser remains a member of our Board of Directors for so long as Mr. Ault beneficially owns no less than 5% of the outstanding shares of our common stock (for no additional remuneration). Additionally, Mr. Ault will hold the position of Founder and Chairman Emeritus and, as such, have the right to nominate an observer to our Board of Directors for a period of five years after the closing date of the initial public offering. Immediately following the closing of the initial public offering in June 2021, we entered into a five-year consulting agreement with Mr. Ault under which he will provide strategic advisory and consulting services to us in consideration for annual fees of $50,000.
Our accounting and finance department use shared office space within the Costa Mesa offices of BitNile.
DPL purchased $10.0 million (2,000,000 shares) of common stock in the initial public offering at $5.00 per share, the same price and on the same terms as other investors in the initial public offering, except that a reduced underwriting discount was paid to the underwriters for the sale of common stock to DPL. Milton C. Ault III, our Founder and Chairman Emeritus, is an executive officer and director of BitNile, as are several other officers and board members of our company.
Future Transactions
Our Board of Directors has adopted a policy whereby any future transactions between our company and any of our subsidiaries, affiliates, officers, directors, principal stockholders or any affiliates of the foregoing will be on terms no less favorable to us than could reasonably be obtained in “arm’s length” transactions with independent third parties, and any such transactions will also be approved by a majority of our disinterested outside directors
Director Independence
The information required by this item regarding director independence is incorporated by reference to the information set forth in Item 10 of this Annual Report on Form 10-K.
| - 76 - |
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Baker Tilly US, LLP serves as our independent registered public accounting firm for the years ended April 30, 2022 and 2021.
Fees and Services
The following table shows the aggregate fees billed to us for professional services by Baker Tilly US, LLP for the years ended April 30, 2022 and 2021:
| 2022 | 2021 | |||||||
| Audit Services | $ | 165,400 | $ | 107,000 | ||||
| Audit Related Services | — | — | ||||||
| Tax Services | 18,600 | — | ||||||
| All Other Services | — | — | ||||||
| Total | $ | 184,000 | $ | 107,000 | ||||
Audit Fee. This category includes the aggregate fees billed for professional services rendered for the audits of our financial statements for the years ended April 30, 2022 and 2021, for the reviews of the interim financial statements during the years ended April 30, 2022 and 2021, and for other services that are normally provided by the independent auditors in connection with statutory and regulatory filings or engagements for the relevant years.
Audit-Related Fees. This category includes the aggregate fees billed in each of the last two years for assurance and related services by the independent auditors that are reasonably related to the performance of the audits or reviews of the financial statements and are not reported above under “Audit Fees,” and generally consist of fees for other engagements under professional auditing standards, accounting and reporting consultations, internal control-related matters, and audits of employee benefit plans.
Tax Fees. This category includes the aggregate fees billed in each of the last two years for professional services rendered by the independent auditors for tax compliance, tax planning and tax advice.
All Other Fees. This category includes the aggregate fees billed in each of the last two years for products and services provided by the independent auditors that are not reported above under “Audit Fees,” “Audit-Related Fees,” or “Tax Fees.”
The Audit Committee’s policy is to pre-approve all services provided by our independent auditors. These services may include audit services, audit-related services, tax services and other services. The Audit Committee may also pre-approve particular services on a case-by-case basis. Our independent auditors are required to report periodically to the Audit Committee regarding the extent of services they provide in accordance with such pre-approval.
| - 77 - |
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
| - 78 - |
| 23.1* | Consent of Baker Tilly US, LLP, Independent Registered Public Accounting Firm. | ||
| 24.1* | Power of Attorney. Reference is made to the signature page hereto. | ||
| 31.1* | Certification of Chief Executive Officer required by Rule 13a-14(a) or Rule 15d-14(a). | ||
| 31.2* | Certification of Chief Financial Officer required by Rule 13a-14(a) or Rule 15d-14(a). | ||
| 32.1** | Certification of Chief Executive and Financial Officer required by Rule 13a-14(b) or Rule 15d-14(b) and Section 1350 of Chapter 63 of Title 18 of the United States Code. | ||
|
101.INS* |
Inline XBRL Instance Document. The instance document does not appear in the Interactive Data File because its XBRL tags are embedded within the Inline XBRL document. | ||
| 101.SCH* | Inline XBRL Taxonomy Extension Schema Document. | ||
| 101.CAL* | Inline XBRL Taxonomy Extension Calculation Linkbase Document. | ||
| 101.DEF* | Inline XBRL Taxonomy Extension Definition Linkbase Document. | ||
| 101.LAB* | Inline XBRL Taxonomy Extension Label Linkbase Document. | ||
| 101.PRE* | Inline XBRL Taxonomy Extension Presentation Linkbase Document. | ||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101). |
*Filed herewith.
** This certification will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent specifically incorporated by reference into such filing.
+ Indicates management contract or compensatory plan.
| ITEM 16. | FORM 10–K SUMMARY |
None.
| - 79 - |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| | ALZAMEND NEURO, INC. | | |
| Date: July 19, 2022 | By: |
/s/ Stephan Jackman Stephan Jackman |
|
| Date: July 19, 2022 | By: |
/s/ Lien T. Escalona Lien T. Escalona |
|
POWER OF ATTORNEY
KNOW ALL BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Stephan Jackman and Henry Nisser, and each of them, as his or her true and lawful attorneys-in-fact and agents, each with the full power of substitution, for him or her and in his or her name, place or stead, in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or their substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on in the capacities and on the dates indicated.
| Name | Title | Date | ||
|
By: /s/ Stephan Jackman Stephan Jackman |
Chief Executive Officer and Director (principal executive officer) |
July 19, 2022 | ||
|
By: /s/ Lien T. Escalona Lien T. Escalona |
Chief Financial Officer (principal financial and accounting officer) |
July 19, 2022 | ||
|
By: /s/ William B. Horne William B. Horne |
Chairman of the Board | July 19, 2022 | ||
|
By: /s/ Henry C.W. Nisser Henry C.W. Nisser |
Executive Vice President, General Counsel and Director |
July 19, 2022 | ||
|
By: /s/ Mark Gustafson Mark Gustafson |
Director | July 19, 2022 | ||
|
By: /s/ Lynne Fahey McGrath, M.P.H., Ph.D. Lynne Fahey McGrath, M.P.H., Ph.D. |
Director |
July 19, 2022
|
||
|
By: /s/ Andrew H. Woo, M.D., Ph.D. Andrew H. Woo, M.D., Ph.D |
Director | July 19, 2022 | ||
|
By: /s/ Jeffrey Oram Jeffrey Oram |
Director | July 19, 2022 |
| - 80 - |
INDEX TO FINANCIAL STATEMENTS
ALZAMEND NEURO, INC.
| Report of
Independent Registered Public Accounting Firm (PCAOB ID |
F-2 |
| Balance Sheets as of April 30, 2022 and 2021 | F-3 |
| Statements of Operations for the years ended April 30, 2022 and 2021 | F-4 |
| Statements of Changes in Stockholders’ Equity for the years ended April 30, 2022 and 2021 | F-5 |
| Statements of Cash Flows for the years ended April 30, 2022 and 2021 | F-6 |
| Notes to Financial Statements | F-7 – F-18 |
| F-1 |
REPORT OF INDEPENDENT REGISTERED ACCOUNTING FIRM
To the Board of Directors and Stockholders of Alzamend Neuro, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Alzamend Neuro, Inc. (the Company) as of April 30, 2022 and 2021, and the related statements of operations, changes in stockholders’ equity and cash flows for the years then ended and the related notes to the financial statements (collectively, the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of April 30, 2022 and 2021, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/
We have served as the Company's auditor since 2019.
July 19, 2022
| F-2 |
ALZAMEND NEURO, INC.
Balance Sheets
| April 30, 2022 | April 30, 2021 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | $ | ||||||
| Prepaid expenses and other current assets | ||||||||
| TOTAL CURRENT ASSETS | ||||||||
| Property, plant and equipment, net | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued liabilities | $ | $ | ||||||
| Related party payable | ||||||||
| Convertible notes, net | ||||||||
| TOTAL CURRENT LIABILITIES | ||||||||
| TOTAL LIABILITIES | $ | $ | ||||||
| COMMITMENTS AND CONTINGENCIES | ||||||||
| STOCKHOLDERS’ EQUITY | ||||||||
| Convertible Preferred stock, $ par value: shares authorized; Series A Convertible Preferred Stock, $ stated value per share, shares designated; and shares issued and outstanding as of April 30, 2022 and April 30, 2021, respectively | ||||||||
| Common stock, $ par value: shares authorized; and shares issued and outstanding as of April 30, 2022 and April 30, 2021, respectively | ||||||||
| Additional paid-in capital | ||||||||
| Note receivable for common stock – related party | ( | ) | ( | ) | ||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| TOTAL STOCKHOLDERS’ EQUITY | ||||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY | $ | $ | ||||||
The accompanying notes are an integral part of these financial statements.
| F-3 |
ALZAMEND NEURO, INC.
Statements of Operations
| For the Year Ended April 30, | ||||||||
| 2022 | 2021 | |||||||
| OPERATING EXPENSES | ||||||||
| Research and development | $ | $ | ||||||
| General and administrative | ||||||||
| Total operating expenses | ||||||||
| Loss from operations | ( | ) | ( | ) | ||||
| OTHER INCOME (EXPENSE), NET | ||||||||
| Gain on extinguishment of debt | ||||||||
| Interest expense | ( | ) | ( | ) | ||||
| Interest expense - related party | ( | ) | ||||||
| Interest income - related party | ||||||||
| Total other expense, net | ( | ) | ( | ) | ||||
| NET LOSS | $ | ( | ) | $ | ( | ) | ||
| Basic and diluted net loss per common share | $ | ( | ) | $ | ( | ) | ||
| Basic and diluted weighted average common shares outstanding | ||||||||
The accompanying notes are an integral part of these financial statements.
| F-4 |
ALZAMEND NEURO, INC.
Statements of Changes in Stockholders’ Equity
Years Ended April 30, 2022 and April 30, 2021
| Series A Convertible | Additional | Note Receivable for | ||||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Paid-In | Common Stock - | Accumulated | ||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Related Party | Deficit | Total | |||||||||||||||||||||||||
| BALANCES, April 30, 2020 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||||||||
| Issuance of common stock, related party, net | - | |||||||||||||||||||||||||||||||
| Stock-based compensation to employees and consultants | - | - | ||||||||||||||||||||||||||||||
| Issuance of common stock, note receivable – related party | - | - | ||||||||||||||||||||||||||||||
| Fair value of warrants issued in connection with convertible notes | - | - | ||||||||||||||||||||||||||||||
| Fair value of warrants issued in connection with convertible notes- related party | - | - | ||||||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||
| BALANCES, April 30, 2021 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||||||||
| Issuance of common stock for restricted stock awards | - | ( | ) | |||||||||||||||||||||||||||||
| Stock-based compensation to employees and consultants | - | - | ||||||||||||||||||||||||||||||
| Issuance of common stock & warrants-related party, net | - | |||||||||||||||||||||||||||||||
| Proceeds from stock option exercise | - | |||||||||||||||||||||||||||||||
| Proceeds from initial public offering, net of underwriters' discounts and commissions and issuance costs of $ | - | |||||||||||||||||||||||||||||||
| Issuance of shares of common stock for conversion of debt | - | |||||||||||||||||||||||||||||||
| Conversion of Series A convertible stock | ( | ) | ( | ) | ( | ) | ||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||||||
| BALANCES, April 30, 2022 | $ | $ | $ | $ | ( | ) | $ | ( | ) | $ | ||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
| F-5 |
ALZAMEND NEURO, INC.
Statements of Cash Flows
| For the Year Ended April 30, | ||||||||
| 2022 | 2021 | |||||||
| Cash flows from operating activities: | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation expense | ||||||||
| Interest expense - debt discount | ||||||||
| Interest expense - debt discount, related party | ||||||||
| Gain on extinguishment of debt | ( | ) | ( | ) | ||||
| Stock-based compensation to employees and consultants | ||||||||
| Non-cash expense from issuance of common stock | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Accounts payable and accrued expenses | ( | ) | ||||||
| Net cash used in operating activities | ( | ) | ( | ) | ||||
| Cash flows from investing activities: | ||||||||
| Proceeds from repayments of notes receivable - related party | ||||||||
| Purchase of machinery | ( | ) | ||||||
| Net cash provided by investing activities | ( | ) | ||||||
| Cash flows from financing activities: | ||||||||
| Proceeds from the issuance of common stock and warrants - related party, net | ||||||||
| Proceeds from stock option exercise | ||||||||
| Payments of related party payable | ( | ) | ( | ) | ||||
| Proceeds from short-term advances, related party | ||||||||
| Proceeds from note payable | ||||||||
| Proceeds from note receivable for common stock – related party | ||||||||
| Proceeds from convertible note payable | ||||||||
| Proceeds from convertible note payable, related party | ||||||||
| Proceeds from initial public offering, net of
underwriters’ discounts and commissions and issuance costs | ||||||||
| Net cash provided by financing activities | ||||||||
| Net increase in cash | ||||||||
| Cash at beginning of period | ||||||||
| Cash at end of period | $ | $ | ||||||
| Supplemental disclosures of cash flow information: | ||||||||
| Non-cash financing activities: | ||||||||
| Conversion of Series A preferred stock | $ | $ | ||||||
| Fair value of warrants issued in connection with initial public offering | $ | $ | ||||||
| Fair value of warrants issued in connection with March 2021 securities purchase
agreement, related party | $ | $ | ||||||
| Fair value of warrants issued in connection with convertible notes payable, related party | $ | $ | ||||||
| Fair value of warrants issued in connection with convertible notes payable | $ | $ | ||||||
| Issuance of common stock in payment of short-term advances, related party | $ | $ | ||||||
| Issuance of common stock on conversion of note | $ | $ | ||||||
| Issuance of common stock in payment of convertible notes payable, related party | $ | $ | ||||||
| Accrued interest payable for common stock | $ | $ | ||||||
The accompanying notes are an integral part of these financial statements.
| F-6 |
ALZAMEND NEURO, INC.
NOTES TO FINANCIAL STATEMENTS
1. DESCRIPTION OF BUSINESS
Alzamend Neuro, Inc. (the “Company” or “Alzamend”), is an early clinical-stage biopharmaceutical company focused on developing novel products for the treatment of neurodegenerative diseases and psychiatric disorders. The Company’s primary focus is Alzheimer’s disease. With two current and future product candidates, Alzamend aims to bring treatments or cures to market at a reasonable cost as quickly as possible. The Company’s current pipeline consists of two novel therapeutic drug candidates (collectively, the “Technology”): (i) a patented ionic cocrystal technology delivering a therapeutic combination of lithium, proline and salicylate, known as AL001, through two royalty-bearing exclusive worldwide licenses from the University of South Florida Research Foundation, Inc., as licensor (the “Licensor”); and (ii) a patented method using a mutant peptide sensitized cell as a cell-based therapeutic vaccine that seeks to restore the ability of a patient’s immunological system to combat Alzheimer’s, known as AL002 or CA022W, through a royalty-bearing exclusive worldwide license from the same Licensor.
The Company is devoting substantially all its efforts towards research and development of its Technology and raising capital. The Company has not generated any product revenue to date. The Company has financed its operations to date primarily through debt financings and through the sale of its common stock, par value $ per share. The Company expects to continue to incur net losses in the foreseeable future.
2. LIQUIDITY, GOING CONCERN AND MANAGEMENT’S PLANS
The accompanying financial
statements have been prepared on the basis that the Company will continue as a going concern. As of April 30, 2022, the Company had cash
of $
In March of 2021, the
Company entered into a securities purchase agreement (the “SPA”) with DPL, a California limited liability company
(“DPL”) and wholly owned subsidiary of BitNile Holdings, Inc. (“BitNile”), a related party, pursuant to which the Company
agreed to sell an aggregate of
shares of common stock for an aggregate of $
The Company expects to continue to incur losses for the foreseeable future and needs to raise additional capital until it is able to generate revenues from operations sufficient to fund its development and commercial operations. However, based on the Company’s current business plan, management believes that the Company’s cash and cash equivalents at April 30, 2022 are sufficient to meet the Company’s anticipated cash requirements during the twelve-month period subsequent to the issuance of the financial statements included in this Annual Report.
3. SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”) and pursuant to the rules and regulations of the Securities and Exchange Commission (the “Commission”).
Accounting Estimates
The preparation of financial statements, in conformity with U.S. GAAP, requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company’s critical accounting policies that involve significant judgment and estimates include research and development, share-based compensation, warrant valuation, and valuation of deferred income taxes. Actual results could differ from those estimates.
| F-7 |
Cash and Cash Equivalents
The Company considers all highly liquid investments with a remaining maturity of three months or less when purchased to be cash equivalents. As of April 30, 2022 and 2021, the Company had no cash equivalents.
Fair Value of Financial Instruments
Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820, Fair Value Measurement, defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the use of unobservable inputs. The fair value hierarchy is based on three levels of inputs that may be used to measure fair value, of which the first two are considered observable and the last is considered unobservable:
Level 1: Quoted prices in active markets for identical assets or liabilities.
Level 2: Inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 assumptions: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities including liabilities resulting from imbedded derivatives associated with certain warrants to purchase common stock.
The fair values of warrants issued in connection with equity or debt issuance are determined using the Black-Scholes valuation model, a “Level 3” fair value measurement, based on the estimated fair value of the underlying common stock, volatility based on the historical volatility data of similar companies, considering the industry, products and market capitalization of such other entities, the expected life based on the remaining contractual term of the conversion option and warrants and the risk free interest rate based on the implied yield available on U.S. Treasury Securities with a maturity equivalent to the warrants’ contractual life.
Income Taxes
The Company determines its income taxes under the asset and liability method. Under the asset and liability approach, deferred income tax assets and liabilities are calculated and recorded based upon the future tax consequences of temporary differences by applying enacted statutory tax rates applicable to future periods for differences between the financial statements carrying amounts and the tax basis of existing assets and liabilities. Generally, deferred income taxes are classified as current or non-current in accordance with the classification of the related asset or liability. Those not related to an asset or a liability are classified as current or non-current depending on the periods in which the temporary differences are expected to reverse. Valuation allowances are provided for significant deferred income tax assets when it is more likely than not that some or all of the deferred tax assets will not be realized. As of April 30, 2022, the Company had fully reserved the net deferred income tax assets by taking a full valuation allowance against these assets.
The Company recognizes tax liabilities by prescribing a minimum probability threshold that a tax position must meet before a financial statement benefit is recognized and also provides guidance on de-recognition, measurement, classification, interest and penalties, accounting in interim periods, disclosure and transition. The minimum threshold is defined as a tax position that is more likely than not to be sustained upon examination by the applicable taxing authority, including resolution of any related appeals or litigation processes, based on the technical merits of the position. The tax benefit to be recognized is measured as the largest amount of benefit that is greater than 50% likely of being realized upon ultimate settlement. To the extent that the final tax outcome of these matters is different than the amount recorded, such differences impact income tax expense in the period in which such determination is made. Interest and penalties, if any, related to accrued liabilities for potential tax assessments are included in income tax expense. U.S. GAAP also requires management to evaluate tax positions taken by the Company and recognize a liability if the Company has taken uncertain tax positions that more likely than not would not be sustained upon examination by applicable taxing authorities. Management of the Company has evaluated tax positions taken by the Company and has concluded that as of April 30, 2022, there were no uncertain tax positions taken, or expected to be taken, that would require recognition of a liability that would require disclosure in the financial statements.
Research and Development Expenses
Research and development costs are expensed as incurred. Research and development costs consist of scientific consulting fees and lab supplies, as well as fees paid to clinical research organizations that conduct certain research and development activities on behalf of the Company.
The Company has acquired and may continue to acquire the rights to develop and commercialize new product candidates from third parties. The upfront payments to acquire licenses, products or rights, as well as any future milestone payments, are immediately recognized as research and development expense provided that there is no alternative future use of the rights in other research and development projects.
| F-8 |
The Company recognizes stock-based compensation expense for stock options on a straight-line basis over the requisite service period and accounts for forfeitures as they occur. The Company’s stock-based compensation costs are based upon the grant date fair value of options estimated using the Black-Scholes option pricing model. To the extent any stock option grants are made subject to the achievement of a performance-based milestone, management evaluates when the achievement of any such performance-based milestone is probable based on the satisfaction of the performance conditions as of the reporting date.
The Company recognizes stock-based compensation expense for restricted stock on a straight-line basis over the requisite service period and accounts for forfeitures as they occur. The Company’s stock-based compensation for restricted stock is based upon the estimated fair value of the Company’s common stock.
The Black-Scholes option pricing model utilizes inputs which are highly subjective assumptions and generally require significant judgment. Certain of such assumptions involve inherent uncertainties and the application of significant judgment. As a result, if factors or expected outcomes change and the Company uses significantly different assumptions or estimates, the Company’s stock-based compensation could be materially different.
Warrants
The Company accounts for stock warrants as either equity instruments, derivative liabilities, or liabilities in accordance with ASC 480, Distinguishing Liabilities from Equity (“ASC 480”) and ASC 815, Derivatives and Hedging (“ASC 815”), depending on the specific terms of the warrant agreement.
During the year ended April 30, 2022, based on the terms of the Company’s warrant agreements, the Company accounted for the warrants as equity instruments as the warrants were indexed to the common stock, required settlement in shares and would be classified as equity under ASC 815.
The Company utilizes FASB ASC Topic No. 260, Earnings per Share. Basic loss per share is computed by dividing loss available to common stockholders by the weighted-average number of common shares outstanding. Diluted loss per share is computed similar to basic loss per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. Diluted loss per common share reflects the potential dilution that could occur if convertible preferred stock, options and warrants were to be exercised or converted or otherwise resulted in the issuance of common stock that then shared in the earnings of the entity.
Since the effects of outstanding options, warrants and convertible preferred stock are anti-dilutive in the periods presented, shares of common stock underlying these instruments have been excluded from the computation of loss per common share.
| F-9 |
| For the Year Ended April 30, | ||||||||
| 2022 | 2021 | |||||||
| Series A convertible preferred stock | ||||||||
| Stock options (1) | ||||||||
| Restricted stock | ||||||||
| Warrants | ||||||||
| Convertible notes | ||||||||
| (1) |
Recent Accounting Standards
From time to time, new accounting pronouncements are issued by the FASB and adopted by the Company as of the specified effective date. Unless otherwise discussed, the impact of recently issued standards that are not yet effective are not expected to have a material impact on the Company’s financial position or results of operations upon adoption.
In October 2020, the FASB issued ASU 2020-10, Codification Improvements to make incremental improvements to GAAP and address stakeholder suggestions, including, among other things, clarifying that the requirement to provide comparative information in the financial statements extends to the corresponding disclosures section. The Company adopted the ASU effective May 1, 2021. The amendments in this update should be applied retrospectively and at the beginning of the period that includes the adoption date. The impact of adopting the ASU was immaterial to the consolidated results of operations, cash flows, financial position, and disclosures.
In December 2019, the FASB issued ASU No. 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes (“ASU 2019-12”), which is intended to simplify various aspects related to accounting for income taxes. ASU 2019-12 removes certain exceptions to the general principles in Topic 740 and also clarifies and amends existing guidance to improve consistent application. This guidance is effective for fiscal years, and interim periods within those fiscal years, beginning after December 15, 2020, with early adoption permitted. The Company adopted ASU 2018-13 as of May 1, 2021. Adoption of this standard had no material impact on the Company’s financial statements and related disclosures.
In August 2020, the FASB issued ASU 2020-06, Debt – Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging – Contracts in Entity’s Own Equity (Subtopic 815-40). This ASU reduces the number of accounting models for convertible debt instruments and convertible preferred stock. As well as amend the guidance for the derivatives scope exception for contracts in an entity’s own equity to reduce form-over-substance-based accounting conclusions. In addition, this ASU improves and amends the related EPS guidance. Early adoption is permitted, but no earlier than fiscal years beginning after December 15, 2020, including interim periods therein. Adoption is either a modified retrospective method or a fully retrospective method of transition. The adoption of this standard on May 1, 2021, did not have a material impact on the Company’s financial position or results of operations.
The Company has considered all other recently issued accounting standards and does not believe the adoption of such standards will have a material impact on its financial statements.
4. NOTE RECEIVABLE, RELATED PARTY, NET
On April 30, 2019, the Company
and Ault Life Science Fund, LLC (“ALSF”), a related party, entered into a securities purchase agreement for the purchase of shares of
the Company’s common stock for a total purchase price of $
5. PREPAID EXPENSES AND OTHER CURRENT ASSETS
Prepaid expenses and other current assets are as follows:
| April 30, 2022 | April 30, 2021 | |||||||
| Prepaid consulting fees | $ | $ | ||||||
| Prepaid insurance | ||||||||
| Other prepaid expenses | ||||||||
| Other receivables | ||||||||
| Total prepaid expenses and other current assets | $ | $ | ||||||
| F-10 |
On June 14, 2021, the Company
purchased directors and officers insurance for twelve months at an annual premium amount of $
6. INCOME TAXES
The following is a geographical breakdown of the Company’s loss before the provision for income taxes:
| April 30, 2022 | April 30, 2021 | |||||||
| Pre-tax loss: | ||||||||
| Federal | $ | ( | ) | $ | ( | ) | ||
| Foreign | ||||||||
| Total pre-tax income (loss) | $ | ( | ) | $ | ( | ) | ||
Significant components of the Company’s deferred tax assets are as follows:
| April 30, 2022 | April 30, 2021 | |||||||
| Deferred income tax asset: | ||||||||
| Net operating loss carryover | $ | $ | ||||||
| Stock compensation | ||||||||
| Total deferred tax asset | ||||||||
| Fixed assets | ( | ) | ||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Deferred income tax asset, net of allowance | $ | $ | ||||||
A reconciliation of the federal statutory income tax rate to the Company’s effective income tax rate for the years ended April 30, 2022 and 2021, is as follows:
| 2022 | 2021 | |||||||
| Tax benefit at U.S. Federal statutory tax rate | % | % | ||||||
| State income tax, net of federal benefit | % | % | ||||||
| Increase (decrease) in tax rate resulting from: | ||||||||
| Change in valuation allowance | - | % | - | % | ||||
| Stock compensation | % | - | % | |||||
| Other | % | - | % | |||||
| Effective tax rate | % | % | ||||||
In assessing the realization
of deferred tax assets, management considers whether it is more likely than not the Company’s deferred tax assets will be realized.
Management considers the scheduled reversal of deferred tax assets, projected future taxable income and tax planning strategies in making
such assessment. Given historical generation of and expected future taxable losses, the Company determined it is not more likely than
not to utilize its deferred tax assets. Therefore, a full valuation allowance was maintained, as of the years ended April 30, 2022 and
2021, of $
At
April 30, 2022, the Company maintained US Federal and state net operating loss (“NOL”) carryovers of approximately $
| F-11 |
The impact of an uncertain income tax position on the income tax return must be recognized at the largest amount that is more likely than not to be sustained upon audit by the relevant taxing authority. An uncertain income tax position will not be recognized if it has less than a 50% likelihood of being sustained. The Company had no uncertain tax positions as of April 30, 2022.
The Company’s policy is to recognize interest and penalties related to income tax matters in the provision for income taxes. As of April 30, 2022, no interest or penalties have been recorded pertaining to uncertain tax positions.
The Company is subject to taxation in the United States and various U.S. state jurisdictions. All tax years remain open to examination by the Internal Revenue Service and relevant state authorities.
On December 27, 2020, the Consolidated Appropriations Act, 2021 (“CAA 2021”) which included a number of provisions including, but not limited to the extension of numerous employment tax credits, the extension of the Section 179D deduction, enhanced business meals deductions, and the deductibility of expenses paid with Paycheck Protection Program loan funds that are forgiven, was signed into law. Accordingly, the effects of the CAA 2021 have been incorporated into the income tax provision for the year ended April 30, 2022. These provisions did not have a material impact on the income tax provision.
2016 Stock Incentive Plan
On April 30, 2016, the Company’s stockholders approved the Company’s 2016 Stock Incentive Plan (the “Plan”). The Plan provides for the issuance of a maximum of shares of common stock to be offered to the Company’s directors, officers, employees, and consultants. On March 1, 2019, the Company’s stockholders approved an additional shares to be available for issuance under the Plan. Options granted under the Plan have an exercise price equal to or greater than the fair value of the underlying common stock at the date of grant and become exercisable based on a vesting schedule determined at the date of grant. The options expire between and years from the date of grant. Restricted stock awards granted under the Plan are subject to a vesting period determined at the date of grant.
2021 Stock Incentive Plan
In February 2021, the Company’s board of directors (the “Board”) adopted, and the stockholders approved, the Alzamend Neuro, Inc. 2021 Stock Incentive Plan (the “2021 Plan”). The 2021 Plan authorizes the grant to eligible individuals of (1) stock options (incentive and non-statutory), (2) restricted stock, (3) stock appreciation rights, or SARs, (4) restricted stock units, and (5) other stock-based compensation.
Stock Subject to the 2021 Plan. The maximum number of shares of common stock that may be issued under the 2021 Plan is 10,000,000 shares, which number will be increased to the extent that compensation granted under the 2021 Plan is forfeited, expires or is settled for cash (except as otherwise provided in the 2021 Plan). Substitute awards (awards made or shares issued by the Company in assumption of, or in substitution or exchange for, awards previously granted, or the right or obligation to make future awards, in each case by a company that the Company acquires or any subsidiary of the Company or with which the Company or any subsidiary combines) will not reduce the shares authorized for grant under the 2021 Plan, nor will shares subject to a substitute award be added to the shares available for issuance or transfer under the 2021 Plan.
Restricted Stock. In May 2021, The awards require continued service to the Company during the vesting period. The vesting provisions of individual awards may vary as approved by the Board. Compensation expense for restricted stock is generally recorded based on its market value on the date of grant and recognized ratably over the associated service and performance period.
Stock Options. All options that the Company grants are granted at the per share fair value on the grant date. Vesting of options differs based on the terms of each option. The Company has valued the options at their date of grant utilizing the Black Scholes option pricing model. As of the date of issuance of these options, there was not an active public market for the Company’s shares. Accordingly, the fair value of the underlying options was determined based on the historical volatility data of similar companies, considering the industry, products and market capitalization of such other entities. The risk-free interest rate used in the calculations is based on the implied yield available on U.S. Treasury issues with an equivalent term approximating the expected life of the options as calculated using the simplified method. The expected life of the options used was based on the contractual life of the option granted. Stock-based compensation is a non-cash expense because the Company settles these obligations by issuing shares of common stock from its authorized shares instead of settling such obligations with cash payments.
| F-12 |
| Outstanding Options | ||||||||||||||||||||
| Shares Available for Grant | Number of Options | Weighted Average Exercise Price | Weighted Average Remaining Contractual Life (years) | Aggregate Intrinsic Value | ||||||||||||||||
| Balance at April 30, 2020 | $ | $ | ||||||||||||||||||
| Increase to plan shares | ||||||||||||||||||||
| Options granted | ( | ) | $ | |||||||||||||||||
| Balance at April 30, 2021 | $ | $ | ||||||||||||||||||
| Options granted | ( | ) | $ | |||||||||||||||||
| Options exercised | - | ( | ) | $ | ||||||||||||||||
| Options cancelled/forfeited | ( | ) | $ | |||||||||||||||||
| Balance at April 30, 2022 | $ | $ | ||||||||||||||||||
| Options vested and expected to vest at April 30, 2022 | $ | $ | ||||||||||||||||||
| Options exercisable at April 30, 2022 | $ | $ | ||||||||||||||||||
The aggregate intrinsic value in the table above represents the total pretax intrinsic value (i.e., the difference between the estimated fair value on the respective date and the exercise price, times the number of shares) that would have been received by the option holders had all option holders exercised their options.
Stock Options Granted to Employees and Consultants
The estimated fair value of stock options granted to employees and consultants during the years ended April 30, 2022 and 2021 were calculated using the Black-Scholes option-pricing model using the following assumptions:
| For the Year Ended April 30, | ||||||||
| 2022 | 2021 | |||||||
| Expected term (in years) | - | |||||||
| Volatility | % | % - % | ||||||
| Risk-free interest rate | % | % - % | ||||||
| Dividend yield | % | % | ||||||
Expected Term: The expected term represents the period that the options granted are expected to be outstanding and is determined using the simplified method (based on the mid-point between the vesting date and the end of the contractual term).
Expected Volatility: The Company uses an average historical stock price volatility of comparable public companies within the biotechnology and pharmaceutical industry that were deemed to be representative of future stock price trends as the Company only has a limited trading history for its common stock. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available.
Risk-Free Interest Rate: The Company based the risk-free interest rate over the expected term of the options based on the constant maturity rate of U.S. Treasury securities with similar maturities as of the date of the grant.
Expected Dividend: The Company has not paid and does not anticipate paying any dividends in the near future. Therefore, the expected dividend yield was zero.
Stock-based compensation related to restricted
stock grants and stock options were $
Performance Contingent Stock Options Granted to Employee
In November 2018, the Board granted performance-based options under the Plan to the Chief Executive Officer. These options have an exercise price of $ per share.
These options have two separate performance triggers for vesting based upon the therapies achieving certain FDA approval milestones within a specified timeframe. By definition, the performance condition in these options can only be achieved after the performance condition of FDA approval has been achieved. As such, the requisite service period is based on the estimated period over which the market condition can be achieved. When a performance goal is deemed to be probable of achievement, time-based vesting and recognition of stock-based compensation expense commences. In the event any of the milestones are not achieved by the specified timelines, such vesting award will terminate and no longer be exercisable with respect to that portion of the shares. The maximum potential expense associated with the performance-contingent awards is $ of general and administrative expense if all of the performance conditions are achieved as stated in the option agreement. Due to the significant risks and uncertainties associated with FDA approvals, as of April 30, 2022, the Company believes that the achievement of the requisite performance conditions is not probable and, as a result, no compensation cost has been recognized for these awards.
| F-13 |
On November 26, 2019, the Board granted performance- and market-contingent awards to certain key employees and a director. These grants were made outside of the Plan. These awards have an exercise price of $1.50 per share. These awards have multiple separate market triggers for vesting based upon either (i) the successful achievement of stepped target closing prices on a national securities exchange for 90 consecutive trading days later than 180 days after the Company’s initial public offering (“IPO”) for its common stock; or (ii) stepped target prices for a change in control transaction. The target prices range from $15 per share to $40 per share. years, the unvested portion of the performance options will be reduced by 25%. Due to the significant risks and uncertainties associated with achieving the market-contingent awards, as of April 30, 2022, the Company believed that the achievement of the requisite performance conditions was not probable and, as a result, no compensation cost has been recognized for these awards.
Performance Contingent Stock Options Granted to Consultants - TAMM Net
On March 23, 2021, the Company issued performance-based stock options to certain team members at TAMM Net, Inc. to purchase an aggregate of shares of common stock at a per share exercise price of $1.50 per share, of which 50% vest upon the completion of Phase I clinical trial for AL001 by March 31, 2022, and the remaining 50% vest upon completion of Phase I clinical trial for AL002 by December 31, 2022. The Company retained TAMM Net, Inc., a consulting firm based in Georgia for project management experienced with good manufacturing practices to lead, develop and manage the Company’s preclinical and clinical efforts, extending from the current status of each product candidate through the exit or commercialization of the technologies that the Company has licensed.
As of April 30, 2022, the Company has completed the Phase I clinical trial of AL001. The Company recognized stock-based compensation related to the completion of the Phase I clinical trial of AL001 by March 31, 2022. Due to the significant risks and uncertainties associated with achieving the completion of Phase I for AL002, as of April 30, 2022, the Company believed that the achievement of the requisite performance conditions was not probable and, as a result, no compensation cost has been recognized for these awards related to AL002.
Performance Contingent Stock Options Granted to Consultants - Other Consultants
On October 14, 2021, the Company issued performance-based stock options to two consultants to purchase an aggregate of 200,000 shares of common stock with an exercise price of $2.42 per share, of which 50,000 vest upon completion of each of the Phase II clinical trials of AL001 for a bipolar indication, AL001 for a PTSD indication, AL001 for a MDD indication and AL002 for an Alzheimer’s indication.
As of April 30, 2022, the Company believed that the achievement of the requisite performance conditions was not probable and, as a result, no compensation cost has been recognized for these awards related to Phase II of AL001 and AL002.
Stock-Based Compensation Expense
| For the Year Ended April 30, | ||||||||
| 2022 | 2021 | |||||||
| Research and development | $ | $ | ||||||
| General and administrative | ||||||||
| Total | $ | $ | ||||||
As of April 30, 2022, total unamortized stock-based compensation expense related to unvested employee and non-employee awards that are expected to vest was $. The weighted-average period over which such stock-based compensation expense will be recognized is approximately years.
8. WARRANTS
Warrant Issuances During 2022
During the year ended April
30, 2022, the Company issued warrants to purchase an aggregate of shares of common stock at an exercise price of $
| (i) | On June 17, 2021, the Company issued a warrant to purchase an aggregate of shares of common stock
at an exercise price equal to $ |
| F-14 |
| (ii) | On July 28, 2021, the Company received from the FDA a “Study May Proceed” letter for a Phase
I study under the Company’s IND application for AL001. Based on the achievement of this milestone, the Company sold an additional
shares of common stock to DPL for $ |
| (iii) | On March 28, 2022, the Company received the full data set from the Phase I clinical trial for AL001.
Based on the achievement of this milestone, on April 28, 2022, under the SPA, the Company sold an additional shares
of its common stock to DPL for $ |
Warrant Issuances During 2021
During the year ended April 30, 2021, the Company issued warrants to purchase an aggregate of 123,000 shares of common stock at an exercise price of $3.00 per share.
| (i) | On August 11, 2020, the Company issued a warrant to purchase an aggregate of 91,667 shares of common stock at an exercise price equal to $3.00 per share of common stock in connection with the issuance of a convertible promissory note in the principal amount of $275,000. Based on the terms of the Company’s warrant agreement, the Company accounted for the warrant as an equity instrument as the warrant is indexed to the Company’s common stock, require settlement in shares and would be classified as equity under ASC 815. |
| (ii) | On August 31, 2020, the Company issued a warrant to purchase an aggregate of 16,667 shares of common stock at an exercise price equal to $3.00 per share of common stock in connection with the issuance of a convertible promissory note, related party in the principal amount of $50,000. Based on the terms of the Company’s warrant agreement, the Company accounted for the warrant as equity instrument as the warrant is indexed to the Company’s common stock, require settlement in shares and would be classified as equity under ASC 815. |
| (iii) | In December 2020, the Company issued a warrant to purchase an aggregate of 14,666 shares of common stock at an exercise price equal to $3.00 per share of common stock in connection with the issuance of a convertible promissory note in the principal amount of $44,000. Based on the terms of the Company’s warrant agreement, the Company accounted for the warrant as equity instruments as the warrant is indexed to the Company’s common stock, require settlement in shares and would be classified as equity under ASC 815. |
The following table summarizes information about common stock warrants outstanding at April 30, 2022:
| Outstanding | Exercisable | ||||||||||||||||||||
| Weighted | |||||||||||||||||||||
| Average | Weighted | Weighted | |||||||||||||||||||
| Remaining | Average | Average | |||||||||||||||||||
| Exercise | Number | Contractual | Exercise | Number | Exercise | ||||||||||||||||
| Price | Outstanding | Life (years) | Price | Exercisable | Price | ||||||||||||||||
| $ | $ | $ | |||||||||||||||||||
| $ | $ | $ | |||||||||||||||||||
| $ | $ | $ | |||||||||||||||||||
| $ | $ | $ | |||||||||||||||||||
| $ - $ | $ | $ | |||||||||||||||||||
The estimated fair value of warrants granted during the years ended April 30, 2022 and 2021, were calculated using the Black-Scholes option-pricing model using the following assumptions:
| For the Year Ended April 30, | |||
| 2022 | 2021 | ||
| Expected term (in years) | |||
| Volatility | |||
| Risk-free interest rate | 2.92% | ||
| Dividend yield | |||
Expected Term: The expected term represents the period that the warrants granted are expected to be outstanding.
Expected Volatility: The Company uses an average historical stock price volatility of comparable public companies within the biotechnology and pharmaceutical industry that were deemed to be representative of future stock price trends as the Company only has a limited trading history for its common stock. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available.
Risk-Free Interest Rate: The Company based the risk-free interest rate over the expected term of the warrants based on the constant maturity rate of U.S. Treasury securities with similar maturities as of the date of the grant.
Expected Dividend: The Company has not paid and does not anticipate paying any dividends in the near future. Therefore, the expected dividend yield was zero.
| F-15 |
9. OTHER RELATED PARTY TRANSACTIONS
In March 2021, the Company
entered into the SPA with DPL pursuant to which the Company agreed to sell an aggregate of shares of common stock for an aggregate
of $
In May 2021, the Board
and Mr. Ault, the Company’s Founder and Chairman Emeritus, agreed to certain arrangements with regard to Board composition and
other matters. Contemporaneously with
On June 15, 2021, DPL, a related party, purchased of the Company’s IPO shares at the public offering price of $ per share.
10. COMMITMENTS AND CONTINGENCIES
Contractual Obligations
On May 1, 2016, the Company entered into a Standard Exclusive License Agreement for AL002 with Sublicensing Terms with Licensor, pursuant to which Licensor granted the Company a royalty bearing exclusive worldwide license limited to the field of Alzheimer’s Immunotherapy and Diagnostics, under United States Patent No. 8,188,046, entitled “Amyloid Beta Peptides and Methods of Use,” filed April 7, 2009 and granted May 29, 2012.
There are certain initial
license fees and milestone payments required to be paid by the Company to the Licensor pursuant to the terms of license agreements. The
license agreements for AL002 require the Company to pay royalty payments of
Original AL001 License:
| Payment | Due Date | Event | |||
| $ | * | Pre-IND meeting | |||
| $ | * | IND application filing | |||
| $ | * | Upon first dosing of patient in a clinical trial | |||
| $ | * | Upon Completion of first clinical trial | |||
| $ | Upon first patient treated in a Phase III clinical trial | ||||
| $ | Upon FDA approval | ||||
| * |
| F-16 |
AL002 License:
| Payment | Due Date | Event | |||
| $ | * | Upon IND application filing | |||
| $ | Upon first dosing of patient in first Phase I clinical trial | ||||
| $ | Upon completion of first Phase I clinical trial | ||||
| $ | Upon completion of first Phase II clinical trial | ||||
| $ | Upon first patient treated in a Phase III clinical trial | ||||
| $ | Upon FDA BLA approval | ||||
The Company has met the pre-IND meeting, IND application filing, and successfully completed the Phase I clinical trial milestones encompassing AL001. If the Company fails to meet a milestone by its specified date, the Licensor may terminate the license agreement.
Licensor was also granted a preemptive right to acquire such shares or other equity securities that may be issued from time to time by the Company while Licensor remains the owner of any equity securities of the Company.
On June 10, 2020, the
Company obtained two (2) additional royalty-bearing exclusive worldwide licenses from the Licensor to a therapy named AL001. One of the
additional licenses is for the treatment of neurodegenerative diseases excluding Alzheimer’s and the other license is for the treatment
of psychiatric diseases and disorders. There are certain license fees and milestone payments required to be paid pursuant to the terms
of the Standard Exclusive License Agreements with Sublicensing Terms, both dated June 10, 2020 and effective as of November 1,
2019, with the Licensor and the University of South Florida (the “June AL001 License Agreements”). Under each of the June
AL001 License Agreements, a royalty payment of
Additional AL001 Licenses:
| Payment | Due Date | Event | |||
| $ | IND application filing | ||||
| $ | Upon first dosing of patient in a clinical trial | ||||
| $ | Upon Completion of first clinical trial | ||||
| $ | Upon first patient treated in a Phase III clinical trial | ||||
| $ | First commercial sale | ||||
| 11. | CONVERTIBLE NOTES |
In February 2021, the
Company entered into a securities purchase agreement with an institutional investor to sell a convertible promissory note in the
aggregate principal amount of $
The fair value of equity
warrants related to the August 2020 and December 2020 convertible promissory note was recorded as a discount to the convertible promissory note with a corresponding increase to additional paid-in
capital. The Company computed the estimated fair value of the warrants using the Black-Scholes option pricing model and, as a result
of this calculation, recorded debt discount in the amount of $
| F-17 |
| 12. | EQUITY TRANSACTIONS |
The Company is authorized to issue shares of Preferred Stock $ par value. The Board has designated shares as the Series A Preferred Shares. The rights, preferences, privileges and restrictions on the remaining authorized shares of Preferred Stock have not been determined. The Board is authorized to create a new series of preferred shares and determine the number of shares, as well as the rights, preferences, privileges and restrictions granted to or imposed upon any series of preferred shares.
Series A Preferred Shares
In connection with the closing of the IPO, all of the outstanding Series A Preferred Shares were converted into shares of common stock. As of April 30, 2022, there were no Series A Preferred Shares or other shares of Preferred Stock issued or outstanding.
Common Stock
On April 30, 2019, the
Company and ALSF entered into a securities purchase agreement for the purchase of
shares of common stock for a total purchase price of $,
or $
per share with
warrants with a -year
life and an exercise price of $
In March 2021, the Company
entered into the SPA with DPL pursuant to which the Company agreed to sell an aggregate of shares of common stock for an aggregate
of $
Finally, the Company agreed
that for a period of 18 months following the date of the payment of the final tranche of $4 million, DPL will have the right to invest
an additional $
On June 17, 2021, the
Company sold an aggregate of
shares of common stock, including
shares pursuant to the underwriter’s exercise of its option to purchase additional shares, each at an offering price of $
per share, for aggregate gross proceeds of approximately $14.4
million. The proceeds from the offering to the Company, net of underwriting discounts and commissions and offering expenses, were $12.9
million. DPL also purchased shares of common stock for $
13. SUBSEQUENT EVENTS
The Company has evaluated subsequent events through the date the financial statements were issued. The Company has determined that there are no such events that warrant disclosure or recognition in the condensed financial statements presented herein.
F-18