
As filed with the Securities and Exchange Commission on August 2, 2023
Registration No. 333-[●]
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
WASHINGTON D.C. 20549
____________________________
FORM
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
____________________________
(Exact name of registrant as specified in its charter)
| 2834 | ||||
| (State or other jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer | ||
| incorporation or organization) | Classification Code Number) | Identification No.) |
(844) 722-6333
(Address, including zip code, and telephone number,
including area code, of registrant’s principal executive offices)
Chief Executive Officer
Alzamend Neuro, Inc.
(Name, address, including zip code, and telephone number,
including area code, of agent for service)
Copies to:
|
Henry C.W. Nisser, Esq. Executive Vice President and General Counsel Alzamend Neuro, Inc. 100 Park Ave., Suite 1658A New York, NY 10017 (646) 650-5044 |
Kenneth A. Schlesinger, Esq. Spencer G. Feldman, Esq. Olshan Frome Wolosky LLP 1325 Avenue of the Americas, 15th Floor New York, NY 10019 (212) 451-2300 |
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If the only securities being registered on this Form are being offered pursuant to dividend or interest reinvestment plans, please check the following box. o
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, other than securities offered only in connection with dividend or interest reinvestment plans, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a registration statement pursuant to General Instruction I.D. or a post-effective amendment thereto that shall become effective upon filing with the Commission pursuant to Rule 462(e) under the Securities Act, check the following box. o
If this Form is a post-effective amendment to a registration statement filed pursuant to General Instruction I.D. filed to register additional securities or additional classes of securities pursuant to Rule 413(b) under the Securities Act, check the following box. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
| Large accelerated filer o | Accelerated filer o |
| Smaller reporting company | |
| Emerging growth company |
If an emerging growth company, indicate
by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial
accounting standards provided to Section 7(a)(2)(B) of the Securities Act.
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities under this prospectus until the registration statement of which it is a part and filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
PRELIMINARY PROSPECTUS
SUBJECT TO COMPLETION, DATED AUGUST 2, 2023

$25,000,000
Common Stock
Preferred Stock
Warrants
Rights
Units
We may offer and sell, from time to time in one or more offerings, any combination of common stock, preferred stock, warrants, rights or units having an aggregate initial offering price not exceeding $25,000,000. The preferred stock, warrants, rights and units may be convertible, exercisable or exchangeable for common stock or preferred stock or other securities of ours.
Each time we sell a particular class or series of securities, we will provide specific terms of the securities offered in a supplement to this prospectus. The prospectus supplement may also add, update or change information in this prospectus. You should read this prospectus and any prospectus supplement, as well as the documents incorporated by reference or deemed to be incorporated by reference into this prospectus, carefully before you invest in any securities.
This prospectus may not be used to offer or sell our securities unless accompanied by a prospectus supplement relating to the offered securities.
Our common stock is presently listed on the Nasdaq Capital Market under the symbol “ALZN.” On July 31, 2023, the last reported sale price of our common stock was $0.453.
These securities may be sold directly by us, through dealers or agents designated from time to time, to or through underwriters or dealers or through a combination of these methods on a continuous or delayed basis. See “Plan of Distribution” in this prospectus. We may also describe the plan of distribution for any particular offering of our securities in a prospectus supplement. If any agents, underwriters or dealers are involved in the sale of any securities in respect of which this prospectus is being delivered, we will disclose their names and the nature of our arrangements with them in a prospectus supplement. The net proceeds we expect to receive from any such sale will also be included in a prospectus supplement.
An investment in our common stock involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” contained on page 11 of this prospectus and in our Annual Report on Form 10-K for the year ended April 30, 2023, as well as our subsequently filed periodic and current reports that we file with the Securities and Exchange Commission and which are incorporated by reference into the registration statement of which this prospectus is a part. We may also include additional risk factors in a prospectus supplement under the heading “Risk Factors.” You should read this prospectus and the applicable prospectus supplement carefully before you make your investment decision.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
This prospectus is dated ________, 2023
TABLE OF CONTENTS
|
Page
| ||
| About this Prospectus | 1 | |
| Disclosure Regarding Forward-Looking Statements | 2 | |
| Prospectus Summary | 3 | |
| Risk Factors | 11 | |
| Use of Proceeds | 12 | |
| Plan of Distribution | 13 | |
| Description of Securities We May Offer Capital Stock | 16 | |
| Description of Capital Stock | 16 | |
| Description of Warrants | 17 | |
| Description of Rights | 19 | |
| Description of Units | 19 | |
| Legal Matters | 20 | |
| Experts | 20 | |
| Where you can find more Information | 20 | |
| Incorporation of Documents by Reference | 21 |
| i |
ABOUT THIS PROSPECTUS
This prospectus is part of a shelf registration statement that we filed with the Securities and Exchange Commission (the “Commission”) using a “shelf” registration process. Under this shelf registration process, we may sell any combination of the securities described in this prospectus in one or more offerings from time to time having an aggregate initial offering price of $25,000,000. This prospectus provides you with a general description of the securities we may offer. Each time we offer securities, we will provide you with a prospectus supplement that describes the specific amounts, prices and terms of the securities we offer. The prospectus supplement also may add, update or change information contained in this prospectus. You should read carefully both this prospectus and any prospectus supplement together with additional information described below under the caption “Where You Can Find More Information.”
This prospectus does not contain all the information provided in the registration statement we filed with the Commission. You should read both this prospectus, including the section titled “Risk Factors,” and the accompanying prospectus supplement, together with the additional information described under the heading “Where You Can Find More Information.”
This prospectus may be supplemented from time to time to add, to update or change information in this prospectus. Any statement contained in this prospectus will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in such prospectus supplement modifies or supersedes such statement. Any statement so modified will be deemed to constitute a part of this prospectus only as so modified, and any statement so superseded will be deemed not to constitute a part of this prospectus. You should rely only on the information contained or incorporated by reference in this prospectus, any applicable prospectus supplement or any related free writing prospectus. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. No dealer, salesperson or other person is authorized to give any information or to represent anything not contained in this prospectus, any applicable prospectus supplement or any related free writing prospectus. This prospectus is not an offer to sell securities, and it is not soliciting an offer to buy securities, in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus or any prospectus supplement, as well as information we have filed with the Commission that is incorporated by reference, is accurate as of the date on the front of those documents only, regardless of the time of delivery of this prospectus or any applicable prospectus supplement, or any sale of a security. Our business, financial condition, results of operations and prospects may have changed since those dates.
No person is authorized in connection with this prospectus to give any information or to make any representations about us, the securities offered hereby or any matter discussed in this prospectus, other than the information and representations contained in this prospectus. If any other information or representation is given or made, such information or representation may not be relied upon as having been authorized by us.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under “Where You Can Find More Information.”
For investors outside the United States: Neither we nor any underwriter has done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus.
Unless otherwise stated or the context requires otherwise, references to “Alzamend,” the “Company,” “we,” “us” or “our” are to Alzamend Neuro, Inc., a Delaware corporation.
| - 1 - |
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference in it contain forward-looking statements regarding future events and our future results that are subject to the safe harbors created under the Securities Act of 1933 and the Securities Exchange Act of 1934. All statements other than statements of historical facts are statements that could be deemed forward-looking statements. These statements are based on our expectations, beliefs, forecasts, intentions and future strategies and are signified by the words “expects,” “anticipates,” “intends,” “believes” or similar language. In addition, any statements that refer to projections of our future financial performance, our anticipated growth, trends in our business and other characterizations of future events or circumstances are forward-looking statements. These forward-looking statements are only predictions and are subject to risks, uncertainties and assumptions that are difficult to predict, including those identified above, under “Risk Factors” and elsewhere in this prospectus. Therefore, actual results may differ materially and adversely from those expressed in any forward-looking statements. All forward-looking statements included in this prospectus are based on information available to us on the date of this prospectus and speak only as of the date hereof.
We disclaim any current intention to update our “forward-looking statements,” and the estimates and assumptions within them, at any time or for any reason. In particular, the following factors, among others, could cause actual results to differ materially from those described in the “forward-looking statements”:
| • | our need for substantial additional funding to finance our operations and complete development to seek FDA approval for AL001 and ALZN002 before commercialization; |
| • | our ability to effectively execute our business strategy; |
| • | our ability to manage our expansion, growth and operating expenses; |
| • | our ability to evaluate and measure our business, prospects and performance metrics; |
| • | our ability to compete and succeed in a highly competitive and evolving industry; |
| • | our ability to respond and adapt to changes in technology and customer behavior; |
| • | our ability to protect our intellectual property and to develop, maintain and enhance a strong brand; |
| • | our significant losses since inception and anticipation that we will continue to incur significant losses for the foreseeable future; |
| • | our reliance on licenses from a third party regarding our rights and development of AL001 and AL002; |
| • | our development of AL001 and AL002 never leading to a marketable product; |
| • | our product candidates not qualifying for expedited development, or if they do, not actually leading to a faster development or regulatory review or approval process; |
| • | our approach to targeting beta-amyloid plaque via AL002 being based on a novel therapeutic approach; and |
| • | the risk factors included in our most recent filings with the SEC, including, but not limited to, our Forms 10-K and 10-Q, which are incorporated by reference herein. |
| - 2 - |
PROSPECTUS SUMMARY
This summary highlights selected information contained in other parts of this prospectus. Because it is a summary, it does not contain all of the information that you should consider in making your investment decision. Before investing in our securities, you should read the entire prospectus carefully, including the information set forth under the heading “Risk Factors.”
Company Overview
We are a clinical-stage biopharmaceutical company focused on developing novel products for the treatment of Alzheimer’s disease (“Alzheimer’s”), bipolar disorder (“BD”), major depressive disorder (“MDD”) and post-traumatic stress disorder (“PTSD”). With our two product candidates, we aim to bring treatments or potential cures to market as quickly as possible. Far too many individuals, patients and caregivers suffer from the burden created by these devastating, and often fatal, diseases. Our primary target, Alzheimer’s, was among the most-feared diseases (second only to cancer) among Americans, according to a 2011 survey by the Harvard School of Public Health. Alzheimer’s is also the seventh leading cause of death in the United States (“U.S.”) according to a 2021 report from the Alzheimer’s Association, a nonprofit that funds research. Existing Alzheimer’s treatments only temporarily relieve symptoms and while one treatment has been shown to slow the progression of the disease, no treatments have been shown to halt the progression of the disease, which currently affects roughly 6.7 million Americans and that number is expected to grow to 13 million individuals by 2050. Alzheimer’s also impacts more than 11 million Americans who provide an estimated 16 billion hours of unpaid care per year, valued at $272 billion, according to data provided by the Alzheimer’s Association. In 2022, the estimated healthcare costs for treating individuals with Alzheimer’s in the U.S. will be $321 billion, including $206 billion in Medicare and Medicaid payments. These costs could rise to as high as $1 trillion per year by 2050 if no permanent treatment or cure for Alzheimer’s is found, the Alzheimer’s Association reported.
Our pipeline consists of two novel therapeutic drug candidates:
| · | AL001 - A patented ionic cocrystal technology delivering a therapeutic combination of lithium, salicylate and proline through three royalty-bearing exclusive worldwide licenses from the University of South Florida Research Foundation, Inc., as licensor (the “Licensor”); and |
| · | ALZN002 - A patented method using a mutant peptide sensitized cell as a cell-based therapeutic vaccine that seeks to restore the ability of a patient’s immunological system to combat Alzheimer’s through a royalty-bearing exclusive worldwide license from the Licensor. |
Our most advanced product candidate (lead product) licensed and in clinical development in humans is AL001, an ionic cocrystal of lithium for the treatment of Alzheimer’s, BD, MDD and PTSD. Based on our preclinical data involving mice models, AL001 treatment prevented cognitive deficits, depression and irritability and is superior in improving associative learning and memory and irritability compared with lithium carbonate treatments, supporting the potential of this lithium formulation for the treatment of Alzheimer’s, BD, MDD and PTSD in humans. Lithium has been marketed for more than 35 years and human toxicology regarding lithium use has been well characterized, potentially mitigating the regulatory burden for safety data.
The results of randomized, placebo-controlled, clinical trials of lithium in the treatment of patients with Alzheimer’s dementia and subjects with mild cognitive impairment have been widely published. Clinical studies have indicated that lithium administered at doses lower than those used for affective disorders can favorably impact Alzheimer’s outcomes. A study by O.V. Forlenza, et al., entitled “Disease-Modifying Properties of Long-Term Lithium Treatment for Amnestic Mild Cognitive Impairment: Randomized Controlled Trial,” appearing in the British Journal of Psychiatry (2011) reported that lithium was superior to a placebo, evidencing a slower decline of cognitive function as measured by the Alzheimer’s Disease Assessment Scale cognitive subscale. Given the absence of adequate widely adapted treatments that can slow, halt or even reverse the decline of this highly prevalent disease, the potential efficacy of lithium in the long-term management of Alzheimer’s may positively impact public health. There is an unmet medical need for safe and effective Alzheimer’s treatments, particularly for treatments with neuroprotective properties.
There is increasing evidence to suggest that depressive illness, particularly in the elderly, is associated with neuronal cell loss. These findings suggest that lithium may exert some of its long-term beneficial effects in the treatment of affective disorders via underappreciated neuroprotective effects. Molecular biology and animal studies have also suggested that lithium may offer protection against Alzheimer’s. Given the absence of other adequate treatments, the potential efficacy of lithium in the long-term treatment of neurodegenerative disorders may be warranted.
| - 3 - |
Our Business Strategy
We intend to develop and commercialize therapeutics that are better than existing treatments and have the potential to significantly improve the lives of individuals afflicted by Alzheimer’s, BD, MDD and PTSD. To achieve these goals, we are pursuing the following key business strategies:
| • | Advance clinical development of AL001 for Alzheimer’s, BD, MDD and PTSD treatment. We completed our Phase I clinical trial in March 2022 and initiated a Phase IIA MAD clinical trial in May 2022. We completed the clinical portion of the Phase IIA Multiple Ascending Dose (“MAD”) clinical trial in March 2023 and reported topline data in June 2023. We intend to initiate two Phase II clinical trials to investigate the safety and efficacy of AL001 for patients with mild to moderate Alzheimer’s. Additionally, we intend to investigate the potential of AL001 for patients suffering from BD, MDD and PTSD by submitting investigational new drug (“IND”) applications to the U.S. Food and Drug Administration (“FDA”) for these indications by the end of 2023. If we achieve successful Phase III clinical trials in humans, we intend to seek approval to commercialize AL001 via a New Drug Application (“NDA”); |
| • | Advance clinical development of ALZN002 for Alzheimer’s treatment. We submitted an IND application to the FDA in September 2022, and received a “study may proceed” letter in October 2022. In April 2023, we initiated a Phase I/IIA clinical trial for ALZN002 to treat mild to moderate dementia of the Alzheimer’s type. If we achieve successful Phase III clinical trials in humans, we intend to seek approval to commercialize ALZN002 via an NDA; |
| • | Expand our pipeline of pharmaceuticals to include additional indications for AL001 and delivery methods. Another element of our business strategy is to expand our pipeline of pharmaceuticals based on our technology and advance these product candidates through clinical development for the treatment of a variety of indications. In addition to treating Alzheimer’s, AL001 has the potential to treat a wide range of neurodegenerative diseases and psychiatric disorders. We plan to pursue the treatment of BD, MDD, and PTSD with AL001, and in May 2022, we submitted a pre-Investigational New Drug (“pre-IND”) meeting request to the FDA for these indications and received a written response from the FDA in July 2022. Based on the written response from the FDA and the receipt of topline data from the Phase IIA MAD clinical trial, we plan to submit separate INDs for BD, MDD, and PTSD by the end of 2023, which, after receipt from the FDA of a “study may proceed” letter for such indication, would allow us to initiate a Phase II study. We also plan to explore different formulations (liquid, immediate release and sprinkle capsules) to deliver AL001; |
| • | Focus on translational and functional endpoints to efficiently develop product candidates. We believe AL001 is positioned for a Section 505(b)(2) regulatory pathway for new drug approvals. We also believe AL001 and ALZN002 are positioned for breakthrough therapy designations because of their positive effects on a pharmacodynamic biomarker (beta-amyloids) and potential for a clinically meaningful effect on Alzheimer’s, making them eligible to receive assistance from the FDA throughout the development process that may shorten the development timelines. However, we have neither received breakthrough therapy designation nor have we qualified for expedited development, and no assurance can be given that we will. Even if we qualify for breakthrough therapy designation or expedited development, it may not actually lead to faster development or expedited regulatory review and approval or necessarily increase the likelihood that we will receive FDA approval; and |
| • | Optimize the value of AL001 and ALZN002 in major markets. We intend to commercialize AL001 and ALZN002 by seeking FDA marketing approval for both product candidates and partnering with biopharmaceutical companies seeking to strategically fortify pipelines and, in turn, receiving funding for the costly later-stage clinical development. We do not anticipate selling products directly into the marketplace, though we may do so depending on market conditions. Our focus is expected to concentrate on entering into strategical transactions with established distributors and producers, which will provide distribution and marketing capabilities for the sale of our products into the marketplace. |
Our Development Pipeline
The following chart provides an overview of the current development stages of our therapeutic product candidates.
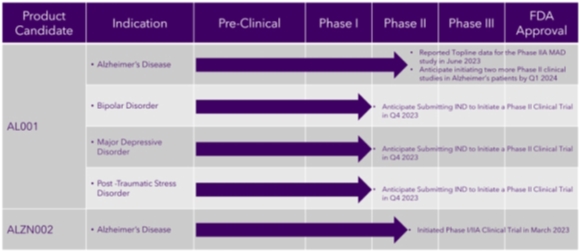
| - 4 - |
Our product candidates will require extensive clinical evaluation, regulatory review and approval, significant marketing efforts and substantial investment before it or any successors are likely to provide us with any revenue. As a result, if we do not successfully develop, achieve regulatory approval for and commercialize our product candidates, our long-term business plans will not be met, and we will be unable to generate the revenue we have forecast for the foreseeable future, if any. We do not anticipate that we will generate our maximum revenue for several years, or that we will achieve profitability for any of our therapeutic drug candidates until at least a few years after generating material revenue, if at all. If we are unable to generate revenue or raise substantial additional capital, we will not be able to pursue any expansion of our business or acquire additional intellectual property, we will not become profitable with our therapeutic drug candidates, and we will be unable to continue our operations at the currently planned pace, if at all.
AL001 Drug Candidate
Our lead product candidate that we have licensed and have begun clinical development in humans is an ionic cocrystal of lithium for the treatment of Alzheimer’s, BD, MDD and PTSD. Lithium salts have a long history of human consumption beginning in the 1800s. In psychiatry, they have been used to treat mania and as a prophylactic for depression since the mid-20th century. Today, lithium salts are used as a mood stabilizer for the treatment of BD. Although the FDA has approved no medications as safe and effective treatments for suicidality, lithium has proven to be the only drug that consistently reduces suicidality in patients with neuropsychiatric disorders. Despite these effective medicinal uses, current FDA-approved lithium pharmaceutics (lithium carbonate and lithium citrate) are limited by a narrow therapeutic window that requires regular blood monitoring of plasma lithium levels and blood chemistry by a clinician to mitigate adverse events. Because conventional lithium salts (carbonate and citrate) are eliminated relatively quickly, multiple administrations throughout the day are required to safely reach therapeutic plasma concentrations. Existing lithium drugs, such as lithium chloride and lithium carbonate, suffer from chronic toxicity, poor physicochemical properties and poor brain bioavailability. Because lithium is so effective at reducing manic episodes in patients with BD, it is still used clinically despite its narrow therapeutic index. This has led researchers to begin to look for alternatives to lithium with similar bioactivities.
Scientists from the University of South Florida have developed a new lithium cocrystal composition and method of preparation that, under certain clinical and/or testing conditions, have been shown to allow for lower dosages to achieve therapeutic brain levels of lithium for psychiatric disorders, which could lead to a broadening of lithium’s therapeutic index. Our studies and/or testing have indicated that the compound offers improved physiochemical properties compared to existing forms of lithium, giving it the potential to be developed as an anti-suicidal drug and for use against mood disorders.
Recent evidence suggests that lithium may be efficacious for both the treatment and prevention of Alzheimer’s. Unlike traditional medications which only address a single therapeutic target, lithium appears to be neuroprotective through several modes of action. For example, recent studies have indicated that it exerts neuroprotective effects, in part, by increasing a brain-derived neurotrophic factor leading to restoration of learning and memory. Another neuroprotective mechanism of lithium indicated by recent studies is the attenuation of the production of inflammatory cytokines like IL-6 and nitric oxide in activated microglia. Results from recent clinical studies suggest that lithium treatment may reduce dementia development while preserving cognitive function and reducing biomarkers associated with Alzheimer’s.
The novel ionic cocrystal of lithium (AL001), which was designed, synthesized and characterized by a team of inventors from the University of South Florida has been shown to exhibit improved nonclinical pharmacokinetics compared to currently FDA-approved lithium products and is also bioactive in many in vitro models of Alzheimer’s. AL001 may constitute a means of treating Alzheimer’s, BD, MDD and PTSD.
We believe that our ability to re-engineer lithium solid dosage forms in order to optimize performance and has the potential to address a wide range of clinical applications ranging from neurodegenerative disorders, not merely Alzheimer’s, but also amyotrophic lateral sclerosis (known as ALS and Lou Gehrig’s disease), Huntington’s disease, multiple sclerosis, Parkinson’s disease and traumatic brain injury, to more psychiatric conditions such as BD, MDD, mania, PTSD and suicidality. This novel approach is intended to achieve the desired therapeutic outcome of enhanced penetration through the blood-brain barrier and sustained brain lithium concentrations while systemic exposures (and toxicities) are mitigated for other organ systems. The optimal modified-release lithium dosing approach for AL001 should avoid acutely toxic peak concentrations in blood, as well as in the brain, and should maintain such blood concentrations for a predictable, clinically relevant time, with overall low systemic exposures that mitigate the potential for adverse events. We anticipate that the lithium delivery system will be adaptable to a dosing regimen that maintains therapeutic brain lithium concentrations consistently for the longest possible time while allowing only modest exposures and providing adequate recovery periods between doses for other organ systems.
| - 5 - |
Clinical Trials
Phase I Study
On September 13, 2021, we initiated a randomized, balanced, Phase I, single-dose, open-label, two-treatment, two-period, two- sequence, crossover, relative bioavailability clinical trial to investigate lithium pharmacokinetics and safety of AL001 formulation compared to a marketed immediate release lithium carbonate formulation in healthy subjects. The primary objective of this clinical trial was to assess the relative bioavailability of the AL001 lithium formulation relative to a marketed lithium carbonate formulation in healthy subjects for the purpose of determining potential clinically safe and effective AL001 dosing in future studies. Additionally, we wanted to characterize safety and tolerability of the tested formulations under the conditions of this clinical trial. This was a first-in-human clinical trial of the AL001 formulation; this trial was designed to assess the relative bioavailability of the AL001 lithium formulation compared to a marketed lithium carbonate formulation in at least 24 completed healthy subjects (30 subjects were to be enrolled) for the purpose of determining potential clinically safe and effective AL001 dosing in future clinical trials. The AL001 lithium content was nearly half of the reference lithium carbonate capsule dosage as it was expected that treatment of frail Alzheimer’s patients will require half the lithium dose used for treatment of BD. Lithium carbonate 300 mg (Reference product) was given as a single dose in this clinical trial; this is often used as a starting dose for treatment of BD when given three times daily. The shape of the AL001 lithium plasma concentration versus time curve was unknown prior to this study. Also unknown were the AL001 rate and extent of lithium absorption. The Phase I study was completed in March 2022 with the following results:
| · | AL001 was shown to be safe and well-tolerated in healthy adult subjects; |
| · | No serious adverse events and no deaths were reported during the trial; |
| · | The safety profiles of both AL001 and the marketed lithium carbonate capsule were benign; |
| · | No clinically significant abnormal findings in electrocardiograms were noted during the trial; |
| · | AL001 salicylate plasma concentrations were observed to be well tolerated and consistently within safe limits; and |
| · | Dose-adjusted relative bioavailability analyses of the rate and extent of lithium absorption in plasma indicated that AL001, at a lithium carbonate equivalent dose of 150 mg, is bioequivalent to a marketed 300 mg lithium carbonate capsule and the shapes of the lithium plasma concentration versus time curves are similar. |
Phase IIA Study
On May 5, 2022, we initiated a multiple-dose, steady-state, double-blind, ascending dose safety, tolerability, pharmacokinetic clinical trial (www.clinicaltrials.gov, identifier: NCT05363293) of AL001 in patients with mild to moderate Alzheimer’s and healthy subjects with the following objectives:
| · | Primary: To evaluate the safety and tolerability of AL001 under multiple-dose, steady-state conditions in Alzheimer’s patients and healthy subjects; |
| · | Secondary: To characterize the maximum tolerated dose (MTD) of AL001 in patients with mild to moderate Alzheimer’s and healthy subjects; and |
| · | Exploratory: Determination of qualitative and quantitative evaluations of AD patient and healthy subjects desirable characteristics for future Phase II and III clinical studies in order to: |
| o | Facilitate recruitment into subsequent AL001 clinical trials; and |
| o | Facilitate trial-adherence to completion of study requirements including treatment adherence. |
We completed the Phase IIA clinical trial in March 2023 and announced positive topline data in June 2023. We announced that we successfully identified a maximum tolerated dose (“MTD”) for development of AL001 from a multiple-ascending dose study as assessed by an independent safety review committee. This dose, providing lithium at a lithium carbonate equivalent dose of 240 mg 3-times daily (“TID”), is designed to be unlikely to require lithium therapeutic drug monitoring (“TDM”). Also, this MTD is risk mitigated for the purpose of treating fragile populations, such as Alzheimer’s patients.
| - 6 - |
Lithium is a commonly prescribed drug for manic episodes in BP type 1 as well as maintenance therapy of BP in patients with a history of manic episodes. Lithium is also prescribed off-label for MDD, BP and treatment of PTSD, among other disorders. Lithium was the first mood stabilizer approved by the FDA and is still a first-line treatment option (considered the “gold standard”) but is underutilized perhaps because of the need for TDM. Lithium was the first drug that required TDM by regulatory authorities in product labelling because the effective and safe range of therapeutic drug blood concentrations is narrow and well defined for treatment of BP when using lithium salts. Excursions above this range can be toxic, and below can impair effectiveness.
Planned Future Studies
Based on the results from our Phase IIA MAD study, we plan to initiate two safety and efficacy clinical trials in subjects with mild to moderate dementia of the Alzheimer’s type. Additionally, we intend to investigate the potential of AL001 for patients suffering from BD, MDD and PTSD by submitting IND applications to the FDA for these indications by the end of 2023. After FDA permission to proceed on the INDs, we intend to initiate clinical trials at this MTD to determine relative increased lithium levels in the brain compared to a marketed lithium salt for BD, MDD and PTSD, based on published mouse studies that predict that lithium can be given at lower doses for equivalent therapeutic benefit when treating with AL001. For example, the goal is to replace a 300 mg TID lithium carbonate dose for treatment of BD with a 240 mg TID AL001 lithium equivalent, which represents a daily decrease of 20% of lithium given to a patient.
ALZN002 Drug Candidate
The other product candidate that we have licensed to clinically develop in humans is ALZN002, a patented method using a mutant peptide sensitized cell as a cell-based therapeutic vaccine which seeks to restore the ability of the patient’s immunological system to combat Alzheimer’s. The proposed mechanism of action is through the pulsed-Dendritic Cell (“DC”) activation of T-cells that stimulates the immune system, resulting in the clearance of brain amyloid. Preclinical studies conducted from April 2005 to July 2010 demonstrated that the infusion of transgenic (or genetically modified) mice with ALZN002-pulsed DCs is associated with lower amyloid burden and improved neuro-behavioral performance. This is likely to be mediated by an anti-inflammatory effect in addition to the immunogenicity of this therapy.
ALZN002 is based on the theory that Alzheimer’s symptoms may be caused in large part by plaque deposits that can cluster in the brain composed of protein fragments called beta-amyloids that build up between nerve cells. One hypothesis is that a special type of immune cell, natural beta-amyloid antibodies, may play a role in preventing plaque build-up in people without Alzheimer’s. As people age, their immune systems may degrade, and some people may be unable to produce natural beta-amyloid antibodies, the absence of which leads to the plaque build-up causing Alzheimer’s.
ALZN002 is intended to elicit an immune response to produce anti-amyloid antibodies, which can then neutralize circulated beta-amyloids and prevent additional plaque build-up. The mutant antigen within ALZN002 was selected specifically for its high Human Leukocyte Antigens (“HLA”) binding affinity, thereby avoiding the need for an adjuvant, which may cause an adverse (Th1) immune response.
ALZN002 is an autologous modified DC treatment. More precisely, it is a patient-specific therapy where the patient undergoes leukapheresis, a nonsurgical treatment used to reduce the quantity of white blood cells in the bloodstream, to isolate peripheral blood monocytes that are subsequently matured into DCs using cytokine therapy (IL4+ GM-CSF) cocktail. The DCs are incubated with a modified amyloid beta (Aβ) peptide to sensitize them, and then administered to the same patient.
Significant evidence has accumulated recently suggesting that immunotherapy is a highly promising modality of treatment in Alzheimer’s. Most current immune-based active investigations are focused on passive immunization by pre-prepared Aβ antibody administration. Active immunization may offer additional or more lasting effects on the clearance of amyloid and a safer approach due to its reliance on autologous immune mechanisms. Further, preliminary evidence suggests a recurrence of the amyloid accumulation after clearance with the immunoglobulins. A prior attempt at engaging the immune system to treat Alzheimer’s was conducted using the immunization with pre-aggregated synthetic Aβ (AN-1792) combined with the immunogenic adjuvant QS-21. The Phase IIA study with AN-1792 was terminated by the FDA due to severe meningoencephalitis in approximately 6% of vaccinated subjects. We believe that this may have been caused by using a QS-21 adjuvant in the vaccine formulation.
Clinical Trials
Pre-Clinical
On July 23, 2021, we announced that Alzamend received positive toxicology results for ALZN002 in a good laboratory practices (“GLP”) toxicology study using a transgenic mouse model of Alzheimer’s. The study was conducted by Charles River Laboratories. ALZN002 is a patented method using a mutant-peptide sensitized cell as a cell-based therapeutic vaccine that seeks to restore the ability of a patient’s immunological system to combat Alzheimer’s.
| - 7 - |
A five-dose GLP study with ALZN002-sensitized cells was completed using a transgenic (or genetically modified) mouse model of Alzheimer’s to investigate the tolerability of ALZN002. Single injections were administered on days 1, 30, 50, 70, and 90. The mice were evaluated for potential toxicity and reversibility of any findings at 75 and 90 days after dosing.
Histopathology results demonstrate that there was no indication of T-cell infiltration or meningoencephalitis suggesting that ALZN002 therapy is safe and tolerable as there were no adverse findings over a 90-day period and 90 days after the last dose. There were no treatment-related mortalities or reports of adverse effects on clinical observations, body weight parameters, organ weight parameters, clinical pathology parameters, gross pathology observations, or histopathologic observations during the main study or the recovery phase.
Modified cell therapies, especially DCs, may provide a safer and more patient-specific active immunization. Ex-vivo modification of DCs as a modality of treatment has been previously used in oncological therapeutics. It has been shown to be relatively safe and capable of engaging the immune system to attack the target tissues with success. Its use in Alzheimer’s therapeutics is relatively recent.
Phase I/II Study
We submitted a pre-IND meeting request for ALZN002 and supporting briefing documents to the Center for Biological Evaluation and Research of the FDA on July 30, 2021. We received a written response relating to the pre-IND from the FDA providing a path for Alzamend’s planned clinical development of ALZN002 on September 30, 2021. The FDA agreed to allow Alzamend to submit an IND to conduct a combined Phase I/II study.
On September 28, 2022, we submitted an IND application to the FDA for ALZN002 and received a “study may proceed” letter on October 31, 2022. The product candidate is an immunotherapy vaccine designed to treat mild to moderate dementia of the Alzheimer’s type. ALZN002 is a proprietary “active” immunotherapy product, which means it is produced by each patient’s immune system. It consists of autologous DCs that are activated white blood cells taken from each individual patient so that they can be engineered outside of the body to attack Alzheimer’s-related amyloid-beta proteins. These DCs are pulsed with a novel amyloid-beta peptide (E22W) designed to bolster the ability of the patient’s immune system to combat Alzheimer’s; the goal being to foster tolerance to treatment for safety purposes while stimulating the immune system to reduce the brain’s beta-amyloid protein burden, resulting in reduced Alzheimer’s signs and symptoms. Compared to passive immunization treatment approaches that use foreign blood products (such as monoclonal antibodies), active immunization with ALZN002 is anticipated to offer a more robust and long-lasting effect on the clearance of amyloid. This could provide a safer approach due to its reliance on autologous immune components, using each individual patient’s own white blood cells rather than foreign cells and/or blood products.
On April 3, 2023, we announced the initiation of a phase I/IIA clinical trial for ALZN002 to treat mild to moderate dementia of the Alzheimer’s type. The purpose of this trial is to assess the safety, tolerability, and efficacy of multiple ascending doses of ALZN002 compared with that of a placebo in 20-30 subjects with mild to moderate morbidity. The primary goal of this clinical trial is to determine an appropriate dose of ALZN002 for treatment of patients with Alzheimer’s in a larger Phase IIB efficacy and safety clinical trial, which Alzamend expects to initiate within three months of receiving data from the initial trial.
The continuation of our current development plans with respect to completing our IND applications and conducting the series of human clinical trials for each of our therapeutics requires us to raise additional capital to fund our operations.
Intellectual Property and Licensing Agreements
On July 2, 2018, we entered into two Standard Exclusive License Agreements with Sublicensing Terms for AL001 with the Licensor and its affiliate, the University of South Florida (the “AL001 Licenses”), pursuant to which the Licensor granted us a royalty bearing exclusive worldwide licenses limited to the field of Alzheimer’s, under U.S. Patent Nos. (i) 9,840,521, entitled “Organic Anion Lithium Ionic Cocrystal Compounds and Compositions”, filed September 24, 2015 and granted December 12, 2017, and (ii) 9,603,869, entitled “Lithium Co-Crystals for Treatment of Neuropsychiatric Disorders”, filed May 21, 2016 and granted March 28, 2017. On February 1, 2019, we entered into the First Amendment to the AL001 Licenses, on March 30, 2021, we entered into the Second Amendment to the AL001 Licenses and on June 8, 2023, we entered into the Third Amendment to the AL001 Licenses (collectively, the “AL001 License Agreements”).
The AL001 License Agreements require that we pay combined royalty payments of 4.5% on net sales of products developed from the licensed technology for AL001. We have already paid an initial license fee of $200,000 for AL001. As an additional licensing fee for the license of the AL001 technologies, the Licensor received 2,227,923 shares of our common stock. Minimum royalties for AL001 License Agreements are $40,000 on the first anniversary of the first commercial sale, $80,000 on the second anniversary first commercial sale and $100,000 on the third anniversary of the first commercial sale and every year thereafter, for the life of the AL001 License Agreements.
| - 8 - |
On May 1, 2016, we entered into a Standard Exclusive License Agreement with Sublicensing Terms for ALZN002 with the Licensor (the “ALZN002 License”), pursuant to which the Licensor granted us a royalty bearing exclusive worldwide license limited to the field of Alzheimer’s Immunotherapy and Diagnostics, under U.S. Patent No. 8,188,046, entitled “Amyloid Beta Peptides and Methods of Use”, filed April 7, 2009 and granted May 29, 2012. On August 18, 2017, we entered into the First Amendment to the ALZN002 License, on May 7, 2018, we entered into the Second Amendment to the ALZN002 License, on January 31, 2019, we entered into the Third Amendment to the ALZN002 License, on January 24, 2020, we entered into the Fourth Amendment to the ALZN002 License, on March 30, 2021, we entered into the Fifth Amendment to the ALZN002 License and on April 17, 2023, we entered into the Sixth Amendment to the ALZN002 License (collectively, the “ALZN002 License Agreement”).
The ALZN002 License Agreement requires us to pay royalty payments of 4% on net sales of products developed from the licensed technology for ALZN002. We have already paid an initial license fee of $200,000 for ALZN002. As an additional licensing fee for the license of ALZN002, the Licensor received 3,601,809 shares of our common stock. Minimum royalties for ALZN002 are $20,000 on the first anniversary of the first commercial sale, $40,000 on the second anniversary first commercial sale and $50,000 on the third anniversary of the first commercial sale and every year thereafter, for the life of the ALZN002 License Agreement.
On November 19, 2019, we entered into two Standard Exclusive License Agreements with Sublicensing Terms for two additional indications of AL001 with the Licensor (the “November AL001 License”), pursuant to which the Licensor granted us a royalty bearing exclusive worldwide licenses limited to the fields of (i) neurodegenerative diseases excluding Alzheimer’s and (ii) psychiatric diseases and disorders. On March 30, 2021, we entered into the First Amendments to the November AL001 License and on April 17, 2023, we entered into the Second Amendments to the November AL001 License (collectively, the “November AL001 License Agreements”).
The November AL001 License Agreements require us to pay royalty payments of 3% on net sales of products developed from the licensed technology for AL001 in those fields. We paid an initial license fee of $20,000 for the additional indications. Minimum royalties for November AL001 License Agreements are $40,000 on the first anniversary of the first commercial sale, $80,000 on the second anniversary first commercial sale and $100,000 on the third anniversary of the first commercial sale and every year thereafter, for the life of the November AL001 License Agreements.
These license agreements have an indefinite term that continue until the later of the date no licensed patent under the applicable agreement remains a pending application or enforceable patent, the end date of any period of market exclusivity granted by a governmental regulatory body, or the date on which the licensee’s obligations to pay royalties expire under the applicable license agreement. Under our various license agreements, if we fail to meet a milestone by its specified date, Licensor may terminate the license agreement. The Licensor was also granted a preemptive right to acquire such shares or other equity securities that may be issued from time to time by us while the Licensor remains the owner of any equity securities of our company.
Additionally, we are required to pay milestone payments on the due dates to the Licensor for the license of the AL001 technologies and for the ALZN002 technology, as follows:
Original AL001 Licenses:
| Payment | Due Date | Event | |||
| $ | 50,000 | * | Completed September 2019 | Pre-IND meeting | |
| $ | 65,000 | * | Completed June 2021 | ND application filing | |
| $ | 190,000 | * | Completed December 2021 | Upon first dosing of patient in a clinical trial | |
| $ | 500,000 | * | Completed March 2022 | Upon Completion of first clinical trial | |
| $ | 1,250,000 | 24 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 10,000,000 | 8 years from the effective date of the agreement | Upon FDA NDA approval | ||
| * | Milestone met and completed |
| - 9 - |
ALZN002 License:
| Payment | Due Date | Event | |||
| $ | 50,000 | * | Upon IND application filing | Upon IND application filing | |
| $ | 50,000 | September 2023 | Upon first dosing of patient in first Phase I clinical trial | ||
| $ | 500,000 | 24 months from completion of first Phase I clinical trial | Upon completion of first Phase II clinical trial | ||
| $ | 1,000,000 | 12 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 10,000,000 | 7 years from the effective date of the agreement | Upon FDA Biologics License Application (“BLA”) approval | ||
| * | Milestone met and completed |
Additional AL001 Licenses:
| Payment | Due Date | Event | |||
| $ | 2,000,000 | 36 months from completion of the first Phase II clinical trial | Upon first patient treated in a Phase III clinical trial | ||
| $ | 16,000,000 | August 1, 2029 | First commercial sale | ||
Corporate Information
Our principal executive offices are located at 3480 Peachtree Road NE, Second Floor, Suite 103, Atlanta, GA 30326, and our telephone number is (844) 722-6333. Our corporate website address is www.alzamend.com. The information contained on or accessible through our website is not a part of this prospectus.
| - 10 - |
RISK FACTORS
Investing in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should carefully consider the risk factors we describe in any prospectus supplement and in any related free writing prospectus for a specific offering of securities, as well as those incorporated by reference into this prospectus and any prospectus supplement. You should also carefully consider other information contained and incorporated by reference in this prospectus and any applicable prospectus supplement, including our financial statements and the related notes thereto incorporated by reference in this prospectus. The risks and uncertainties described in the applicable prospectus supplement and our other filings with the Commission incorporated by reference herein are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently consider immaterial may also adversely affect us. If any of the described risks occur, our business, financial condition or results of operations could be materially harmed. In such case, the value of our securities could decline and you may lose all or part of your investment.
| - 11 - |
USE OF PROCEEDS
Except as otherwise provided in the applicable prospectus supplement, we intend to use the net proceeds from the sale of the securities offered by this prospectus for general corporate purposes, which may include working capital, capital expenditures, research and development expenditures, regulatory affairs expenditures, clinical trial expenditures, acquisitions of new technologies and investments, the financing of possible acquisitions or business expansions, and the repayment, refinancing, redemption or repurchase of future indebtedness or capital stock.
The intended application of proceeds from the sale of any particular offering of securities using this prospectus will be described in the accompanying prospectus supplement relating to such offering. The precise amount and timing of the application of these proceeds will depend on our funding requirements and the availability and costs of other funds.
| - 12 - |
PLAN OF DISTRIBUTION
We may sell the securities from time to time to or through underwriters or dealers, through agents, or directly to one or more purchasers. A distribution of the securities offered by this prospectus may also be effected through the issuance of derivative securities, including without limitation, warrants, rights to purchase and subscriptions. In addition, the manner in which we may sell some or all of the securities covered by this prospectus includes, without limitation, through:
| ● | a block trade in which a broker-dealer will attempt to sell as agent, but may position or resell a portion of the block, as principal, in order to facilitate the transaction; |
| ● | purchases by a broker-dealer, as principal, and resale by the broker-dealer for its account; or |
| ● | ordinary brokerage transactions and transactions in which a broker solicits purchasers. |
A prospectus supplement or supplements with respect to each series of securities will describe the terms of the offering, including, to the extent applicable:
● the terms of the offering;
● the name or names of the underwriters or agents and the amounts of securities underwritten or purchased by each of them, if any;
● the public offering price or purchase price of the securities or other consideration therefor, and the proceeds to be received by us from the sale;
● any delayed delivery requirements;
● any over-allotment options under which underwriters may purchase additional securities from us;
● any underwriting discounts or agency fees and other items constituting underwriters’ or agents’ compensation
● any discounts or concessions allowed or re-allowed or paid to dealers; and
● any securities exchange or market on which the securities may be listed.
The offer and sale of the securities described in this prospectus by us, the underwriters or the third parties described above may be effected from time to time in one or more transactions, including privately negotiated transactions, either:
● at a fixed price or prices, which may be changed;
● in an “at the market” offering within the meaning of Rule 415(a)(4) of the Securities Act of 1933, as amended, or the Securities Act;
● at prices related to such prevailing market prices; or
● at negotiated prices.
Only underwriters named in the prospectus supplement will be underwriters of the securities offered by the prospectus supplement.
Underwriters and Agents; Direct Sales
If underwriters are used in a sale, they will acquire the offered securities for their own account and may resell the offered securities from time to time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate.
Unless the prospectus supplement states otherwise, the obligations of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement. Subject to certain conditions, the underwriters will be obligated to purchase all of the securities offered by the prospectus supplement, other than securities covered by any over-allotment option. Any public offering price and any discounts or concessions allowed or re-allowed or paid to dealers may change from time to time. We may use underwriters with whom we have a material relationship. We will describe in the prospectus supplement, naming the underwriter, the nature of any such relationship.
| - 13 - |
We may sell securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities, and we will describe any commissions we will pay the agent in the prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
Dealers
We may sell the offered securities to dealers as principals. The dealer may then resell such securities to the public either at varying prices to be determined by the dealer or at a fixed offering price agreed to with us at the time of resale.
Institutional Purchasers
We may authorize agents, dealers or underwriters to solicit certain institutional investors to purchase offered securities on a delayed delivery basis pursuant to delayed delivery contracts providing for payment and delivery on a specified future date. The applicable prospectus supplement or other offering materials, as the case may be, will provide the details of any such arrangement, including the offering price and commissions payable on the solicitations.
We will enter into such delayed contracts only with institutional purchasers that we approve. These institutions may include commercial and savings banks, insurance companies, pension funds, investment companies and educational and charitable institutions.
Indemnification; Other Relationships
We may provide agents, underwriters, dealers and remarketing firms with indemnification against certain civil liabilities, including liabilities under the Securities Act, or contribution with respect to payments that the agents or underwriters may make with respect to these liabilities. Agents, underwriters, dealers and remarketing firms, and their affiliates, may engage in transactions with, or perform services for, us in the ordinary course of business. This includes commercial banking and investment banking transactions.
Market-Making; Stabilization and Other Transactions
There is currently no market for any of the offered securities, other than our common stock, which is quoted on the Nasdaq Capital Market. If the offered securities are traded after their initial issuance, they may trade at a discount from their initial offering price, depending upon prevailing interest rates, the market for similar securities and other factors. While it is possible that an underwriter could inform us that it intends to make a market in the offered securities, such underwriter would not be obligated to do so, and any such market-making could be discontinued at any time without notice. Therefore, no assurance can be given as to whether an active trading market will develop for the offered securities. We have no current plans for listing of the preferred stock, warrants or subscription rights on any securities exchange or quotation system; any such listing with respect to any particular preferred stock, warrants or subscription rights will be described in the applicable prospectus supplement or other offering materials, as the case may be.
Any underwriter may engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the Securities Exchange Act of 1934, as amended, or the Exchange Act. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum price. Syndicate-covering or other short-covering transactions involve purchases of the securities, either through exercise of the over-allotment option or in the open market after the distribution is completed, to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
| - 14 - |
Any underwriters or agents that are qualified market makers on the Nasdaq Capital Market may engage in passive market making transactions in our common stock on the Nasdaq Capital Market in accordance with Regulation M under the Exchange Act, during the business day prior to the pricing of the offering, before the commencement of offers or sales of our common stock. Passive market makers must comply with applicable volume and price limitations and must be identified as passive market makers. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for such security; if all independent bids are lowered below the passive market maker’s bid, however, the passive market maker’s bid must then be lowered when certain purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Fees and Commissions
If 5% or more of the net proceeds of any offering of securities made under this prospectus will be received by a FINRA member participating in the offering or affiliates or associated persons of such FINRA member, the offering will be conducted in accordance with FINRA Rule 5121.
| - 15 - |
DESCRIPTION OF SECURITIES WE MAY OFFER
The descriptions of the securities contained in this prospectus, together with the applicable prospectus supplements, summarize all the material terms and provisions of the various types of securities that we may offer. We will describe in the applicable prospectus supplement relating to any securities the particular terms of the securities offered by that prospectus supplement. If we indicate in the applicable prospectus supplement, the terms of the securities may differ from the terms we have summarized below. We will also include in the prospectus supplement information, where applicable, about material United States federal income tax considerations relating to the securities, and the securities exchange, if any, on which the securities will be listed.
We may sell from time to time, in one or more offerings:
| • | shares of our common stock; |
| • | shares of our preferred stock; |
| • | warrants to purchase shares of our common stock or preferred stock; |
| • | rights to purchase shares of our common stock; and/or |
| • | units consisting of any of the securities listed above. |
The terms of any securities we offer will be determined at the time of sale. We may issue securities that are exchangeable for or convertible into common stock or any of the other securities that may be sold under this prospectus. When particular securities are offered, a supplement to this prospectus will be filed with the Commission, which will describe the terms of the offering and sale of the offered securities.
DESCRIPTION OF CAPITAL STOCK
The summary does not purport to be complete and is qualified in its entirety by reference to our certificate of incorporation and bylaws, and to the provisions of the General Corporation Law of the State of Delaware, as amended.
We are authorized to issue 300,000,000 shares of common stock, par value $0.0001 per share. As of the date of this prospectus, there were 96,427,624 shares of our common stock issued and outstanding. The outstanding shares of our common stock are validly issued, fully paid and nonassessable. We are authorized to issue up to 10,000,000 shares of preferred stock, par value $0.0001 per share. Of these shares of preferred stock, 1,360,000 are designated as Series A Convertible Preferred Stock. As of the date of this prospectus, there were no shares of Series A Convertible Preferred Stock issued or outstanding.
Common Stock
Holders of our shares of common stock are entitled to one vote for each share on all matters submitted to a shareholder vote. Holders of our common stock do not have cumulative voting rights. Therefore, holders of a majority of the shares of our common stock voting for the election of directors can elect all of the directors. Holders of our common stock representing a majority of the voting power of our capital stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of shareholders. A vote by the holders of a majority of our outstanding shares is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to our certificate of incorporation.
Holders of our common stock are entitled to share in all dividends that our Board of Directors, in its discretion, declares from legally available funds. In the event of a liquidation, dissolution or winding up, each outstanding share entitles its holder to participate pro rata in all assets that remain after payment of liabilities and after providing for each class of stock, if any, having preference over our common stock. Our common stock has no preemptive, subscription or conversion rights and there are no redemption provisions applicable to our common stock.
Preferred Stock
The shares of preferred stock may be issued in series, and shall have such voting powers, full or limited, or no voting powers, and such designations, preferences and relative participating, optional or other special rights, and qualifications, limitations or restrictions thereof, as shall be stated and expressed in the resolution or resolutions providing for the issuance of such stock adopted from time to time by the board of directors. The board of directors is expressly vested with the authority to determine and fix in the resolution or resolutions providing for the issuances of preferred stock the voting powers, designations, preferences and rights, and the qualifications, limitations or restrictions thereof, of each such series to the full extent now or hereafter permitted by the laws of the State of Delaware.
| - 16 - |
The authorized shares of preferred stock will be available for issuance without further action by our stockholders unless such action is required by applicable law or the rules of any stock exchange or automated quotation system on which our securities may be listed or traded. The Nasdaq Stock Market currently requires stockholder approval as a prerequisite to listing shares in several circumstances, including, in certain circumstances, where the issuance of shares could result in an increase in the number of shares of common stock outstanding, or in the amount of voting securities outstanding, of at least 20%.
Transfer Agent and Registrar
The Transfer Agent and Registrar for our common stock is Computershare Trust Company, N.A., 8742 Lucent Blvd., Suite 225, Highlands Ranch, CO 80129.
DESCRIPTION OF WARRANTS
The following description, together with the additional information we may include in any applicable prospectus supplements, summarizes the material terms and provisions of the warrants that we may offer under this prospectus and the related warrant agreements and warrant certificates. While the terms summarized below will apply generally to any warrants that we may offer, we will describe the particular terms of any series of warrants in more detail in the applicable prospectus supplement. If we indicate in the prospectus supplement, the terms of any warrants offered under that prospectus supplement may differ from the terms described below. If there are differences between that prospectus supplement and this prospectus, the prospectus supplement will control. Thus, the statements we make in this section may not apply to a particular series of warrants. Specific warrant agreements will contain additional important terms and provisions and will be incorporated by reference as an exhibit to the registration statement which includes this prospectus.
General
We may issue warrants for the purchase of common stock and/or preferred stock in one or more series. We may issue warrants independently or together with common stock and/or preferred stock, and the warrants may be attached to or separate from these securities.
We will evidence each series of warrants by warrant certificates that we may issue under a separate agreement. We may enter into the warrant agreement with a warrant agent. Each warrant agent may be a bank that we select which has its principal office in the United States and a combined capital and surplus of at least $50,000,000. We may also choose to act as our own warrant agent. We will indicate the name and address of any such warrant agent in the applicable prospectus supplement relating to a particular series of warrants.
We will describe in the applicable prospectus supplement the terms of the series of warrants, including:
| • | the offering price and aggregate number of warrants offered; |
| • | the currency for which the warrants may be purchased; |
| • | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| • | if applicable, the date on and after which the warrants and the related securities will be separately transferable; |
| • | in the case of warrants to purchase common stock or preferred stock, the number of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which these shares may be purchased upon such exercise; |
| • | the warrant agreement under which the warrants will be issued; |
| • | the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreement and the warrants; |
| • | anti-dilution provisions of the warrants, if any; |
| • | the terms of any rights to redeem or call the warrants; |
| • | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
| - 17 - |
| • | the dates on which the right to exercise the warrants will commence and expire or, if the warrants are not continuously exercisable during that period, the specific date or dates on which the warrants will be exercisable; |
| • | the manner in which the warrant agreement and warrants may be modified; |
| • | the identities of the warrant agent and any calculation or other agent for the warrants; |
| • | federal income tax consequences of holding or exercising the warrants; |
| • | the terms of the securities issuable upon exercise of the warrants; |
| • | any securities exchange or quotation system on which the warrants or any securities deliverable upon exercise of the warrants may be listed; and |
| • | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including in the case of warrants to purchase common stock or preferred stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any.
Exercise of Warrants
Each warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants at any time up to 5:00 p.m. Eastern Time on the expiration date that we set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
Holders of the warrants may exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information, and paying the required amount to the warrant agent in immediately available funds, as provided in the applicable prospectus supplement. We will set forth on the reverse side of the warrant certificate, and in the applicable prospectus supplement, the information that the holder of the warrant will be required to deliver to the warrant agent.
Until the warrant is properly exercised, no holder of any warrant will be entitled to any rights of a holder of the securities purchasable upon exercise of the warrant.
Upon receipt of the required payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will issue and deliver the securities purchasable upon such exercise. If fewer than all of the warrants represented by the warrant certificate are exercised, then we will issue a new warrant certificate for the remaining amount of warrants. If we so indicate in the applicable prospectus supplement, holders of the warrants may surrender securities as all or part of the exercise price for warrants.
Enforceability of Rights by Holders of Warrants
Any warrant agent will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action its right to exercise, and receive the securities purchasable upon exercise of, its warrants in accordance with their terms.
Warrant Agreement Will Not Be Qualified Under the Trust Indenture Act
No warrant agreement will be qualified as an indenture, and no warrant agent will be required to qualify as a trustee, under the Trust Indenture Act. Therefore, holders of warrants issued under a warrant agreement will not have the protection of the Trust Indenture Act with respect to their warrants.
| - 18 - |
Governing Law
Each warrant agreement and any warrants issued under the warrant agreements will be governed by New York law.
Calculation Agent
Calculations relating to warrants may be made by a calculation agent, an institution that we appoint as our agent for this purpose. The prospectus supplement for a particular warrant will name the institution that we have appointed to act as the calculation agent for that warrant as of the original issue date for that warrant. We may appoint a different institution to serve as calculation agent from time to time after the original issue date without the consent or notification of the holders.
The calculation agent’s determination of any amount of money payable or securities deliverable with respect to a warrant will be final and binding in the absence of manifest error.
DESCRIPTION OF RIGHTS
This section describes the general terms of the rights that we may offer and sell by this prospectus. This prospectus and any accompanying prospectus supplement will contain the material terms and conditions for each right. The accompanying prospectus supplement may add, update or change the terms and conditions of the rights as described in this prospectus.
The particular terms of each issue of rights, the rights agreement relating to the rights and the rights certificates representing rights will be described in the applicable prospectus supplement, including, as applicable:
| • | the title of the rights; |
| • | the date of determining the stockholders entitled to the rights distribution; |
| • | the title, aggregate number of shares of common stock or preferred stock purchasable upon exercise of the rights; |
| • | the exercise price; |
| • | the aggregate number of rights issued; |
| • | the date, if any, on and after which the rights will be separately transferable; |
| • | the date on which the right to exercise the rights will commence and the date on which the right will expire; and |
| • | any other terms of the rights, including terms, procedures and limitations relating to the distribution, exchange and exercise of the rights. |
DESCRIPTION OF UNITS
We may issue units comprised of one or more of the other securities described in this prospectus in any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
The applicable prospectus supplement will describe:
| • | the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| • | any unit agreement under which the units will be issued; |
| • | any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and |
| • | whether the units will be issued in fully registered or global form. |
The applicable prospectus supplement will describe the terms of any units. The preceding description and any description of units in the applicable prospectus supplement does not purport to be complete and is subject to and is qualified in its entirety by reference to the unit agreement and, if applicable, collateral arrangements and depositary arrangements relating to such units.
| - 19 - |
LEGAL MATTERS
The validity of the securities offered by this prospectus is being passed upon for us by our counsel, Olshan Frome Wolosky LLP, New York, New York. If the securities are distributed in an underwritten offering, certain legal matters will be passed upon for the underwriters by counsel identified in the applicable prospectus supplement.
EXPERTS
The financial statements of Alzamend Neuro, Inc. as of April 30, 2023 and 2022 and for each of the two years in the period ended April 30, 2023 incorporated by reference in this Prospectus and Registration Statement from our Annual Report on Form 10-K for the years ended April 30, 2023 and 2022, have been audited by Baker Tilly US, LLP, an independent registered public accounting firm, as stated in their report thereon (which report expresses an unqualified opinion and includes an explanatory paragraph relating to the Company’s ability to continue as a going concern), incorporated herein by reference, and have been incorporated in this Prospectus and Registration Statement in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the Commission a registration statement on Form S-3 under the Securities Act, with respect to the securities covered by this prospectus. This prospectus and any prospectus supplement which form a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information with respect to us and the securities covered by this prospectus, please see the registration statement and the exhibits filed with the registration statement. Any statements made in this prospectus or any prospectus supplement concerning legal documents are not necessarily complete and you should read the documents that are filed as exhibits to the registration statement or otherwise filed with the Commission for a more complete understanding of the document or matter. A copy of the registration statement and the exhibits filed with the registration statement may be inspected without charge at the Public Reference Room maintained by the Commission, located at 100 F Street, N.E., Washington, D.C. 20549. Please call the Commission at 1-800-SEC-0330 for more information about the operation of the Public Reference Room. The Commission also maintains an internet website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the Commission. The address of the website is http://www.sec.gov.
We file annual, quarterly and current reports, proxy statements and other information with the Commission. You may read, without charge, and copy the documents we file at the Commission’s public reference room in Washington, D.C. at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of these documents by writing to the Commission and paying a fee for the copying cost. Please call the Commission at 1-800-SEC-0330 for further information on the public reference rooms. Our filings with the Commission are available to the public at no cost from the Commission’s website at http://www.sec.gov.
| - 20 - |
INCORPORATION OF DOCUMENTS BY REFERENCE
We are “incorporating by reference” in this prospectus certain documents we file with the Commission, which means that we can disclose important information to you by referring you to those documents. The information in the documents incorporated by reference is considered to be part of this prospectus. Statements contained in documents that we file with the Commission and that are incorporated by reference in this prospectus will automatically update and supersede information contained in this prospectus, including information in previously filed documents or reports that have been incorporated by reference in this prospectus, to the extent the new information differs from or is inconsistent with the old information. We have filed the following document with the Commission, which is incorporated herein by reference as of its date of filing:
| • | Our Annual Report on Form 10-K for the period ended April 30, 2023, filed with the Commission on July 27, 2023. |
All documents that we filed with the Commission pursuant to Sections 13(a), 13(c), 14, and 15(d) of the Exchange Act subsequent to the date of this registration statement and prior to the filing of a post-effective amendment to this registration statement that indicates that all securities offered under this prospectus have been sold, or that deregisters all securities then remaining unsold, will be deemed to be incorporated in this registration statement by reference and to be a part hereof from the date of filing of such documents.
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus shall be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus, or in any subsequently filed document that also is deemed to be incorporated by reference in this prospectus, modifies, supersedes or replaces such statement. Any statement so modified, superseded or replaced shall not be deemed, except as so modified, superseded or replaced, to constitute a part of this prospectus. None of the information that we disclose under Items 2.02 or 7.01 of any Current Report on Form 8-K or any corresponding information, either furnished under Item 9.01 or included as an exhibit therein, that we may from time to time furnish to the Commission will be incorporated by reference into, or otherwise included in, this prospectus, except as otherwise expressly set forth in the relevant document. Subject to the foregoing, all information appearing in this prospectus is qualified in its entirety by the information appearing in the documents incorporated by reference.
You may request, orally or in writing, a copy of these documents, which will be provided to you at no cost (other than exhibits, unless such exhibits are specifically incorporate by reference), by contacting Stephan Jackman, c/o Alzamend Neuro, Inc., at 3480 Peachtree Road NE, Second Floor, Suite 103, Atlanta, GA 30326. Our telephone number is (844) 722-6333. Information about us is also available at our website at www.alzamend.com. However, the information on our website is not a part of this prospectus and is not incorporated by reference.
| - 21 - |
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 14. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION.
The Company is paying all expenses of the offering. The following table sets forth all expenses to be paid by the registrant. All amounts shown are estimates except for the registration fee.
| SEC registration fee | $ | 2,755.00 | ||
| Printing | * | |||
| Legal fees and expenses | $ | * | ||
| Accounting fees and expenses | $ | * | ||
| Trustees’ Fees and Expenses | * | |||
| Warrant Agent Fees and Expenses | * | |||
| Miscellaneous | * | |||
| Total | $ | 2,755.00 |
| * | These fees are calculated based on the securities offered and the number of issuances and accordingly cannot be estimated at this time. The applicable prospectus supplement will set forth the estimated amount of expenses of any offering of securities. |
ITEM 15. INDEMNIFICATION OF DIRECTORS AND OFFICERS.
Section 145 of the Delaware General Corporation Law (the “DGCL”) empowers a Delaware corporation to indemnify any persons who are, or are threatened to be made, parties to any threatened, pending, or completed legal action, suit, or proceeding, whether civil, criminal, administrative, or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person was an officer or director of such corporation, or is or was serving at the request of such corporation as a director, officer, employee, or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines, and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit, or proceeding, provided that such officer or director acted in good faith and in a manner he reasonably believed to be in or not opposed to the corporation’s best interests, and, for criminal proceedings, had no reasonable cause to believe his conduct was illegal. A Delaware corporation may indemnify officers and directors in an action by or in the right of the corporation under the same conditions, except that no indemnification is permitted without judicial approval if the officer or director is adjudged to be liable to the corporation in the performance of his duty. Where an officer or director is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him against the expenses which such officer or director actually and reasonably incurred.
Our bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by Delaware law, except that no indemnification will be provided to a director, officer, employee, or agent if the indemnification sought is in connection with a proceeding initiated by such person without the authorization of our board of directors. The bylaws also provide that the right of directors and officers to indemnification shall be a contract right and shall not be exclusive of any other right now possessed or hereafter acquired under any statute, provision of the certificate of incorporation, bylaw, agreement, vote of stockholders or disinterested directors or otherwise. The bylaws also permit us to secure insurance on behalf of any officer, director, employee, or other agent for any liability arising out of his or her actions in such capacity, regardless of whether the bylaws would permit indemnification of any such liability.
In accordance with Section 102(b)(7) of the DGCL, our certificate of incorporation provides that directors shall not be personally liable for monetary damages for breaches of their fiduciary duty as directors except for (i) breaches of their duty of loyalty to us or our stockholders, (ii) acts or omissions not in good faith or which involve intentional misconduct or knowing violations of law, (iii) certain transactions under Section 174 of the DGCL (unlawful payment of dividends or unlawful stock purchases or redemptions), or (iv) transactions from which a director derives an improper personal benefit. The effect of this provision is to eliminate the personal liability of directors for monetary damages or actions involving a breach of their fiduciary duty of care, including any actions involving gross negligence.
In addition, we have entered into indemnification agreements with our directors and officers that require us, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service, so long as the indemnitee acted in good faith and in a manner the indemnitee reasonably believed to be in or not opposed to the best interests of the Registrant, and, with respect to any criminal action or proceeding, the indemnitee had no reasonable cause to believe his or her conduct was unlawful. We also maintain director and officer liability insurance to insure our directors and officers against the cost of defense, settlement or payment of a judgment under specified circumstances.
| - II-1 - |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the Registrant pursuant to the foregoing provisions, the Registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
ITEM 16. EXHIBITS
| Exhibit Number | Description |
| 1.1** | Form of Underwriting Agreement. |
| 4.1** | Form of Preferred Stock Certificate and Form of Certificate of Designation of Preferred Stock. |
| 4.2** | Form of Warrant Agreement and Form of Warrant Certificate. |
| 4.3** | Form of Rights Agreement and Form of Rights Certificate. |
| 4.4** | Form of Unit Agreement and Unit Certificate. |
| 5.1* | Opinion of Olshan Frome Wolosky LLP, counsel to the Registrant. |
| 23.1* | Consent of Olshan Frome Wolosky LLP (included in Exhibit 5.1). |
| 23.2* | Consent of Baker Tilly US, LLP. |
| 24.1* | Power of Attorney (contained on signature page). |
| 107* | Calculation of Filing Fee Table. |
| * | Filed herewith. |
| ** | If applicable, to be filed by an amendment or as an exhibit to a report pursuant to section 13(a) or section 15(d) of the Exchange Act and incorporated by reference. |
ITEM 17. UNDERTAKINGS.
| (a) | The undersigned registrant hereby undertakes: |
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of this registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in this registration statement. Notwithstanding the foregoing, any increase or decrease in the volume of securities offered (if the total dollar value of the securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in this registration statement or any material change to such information in this registration statement; |
provided, however, that the undertakings set forth in paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) that are incorporated by reference in this registration statement or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of this registration statement;
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
| - II-2 - |
(4) That, for the purpose of determining liability under the Securities Act to any purchaser:
| (i) | If the registrant is relying on Rule 430B; |
| (A) | Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of this registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| (B) | Each prospectus required to be filed pursuant to Rule 424 (b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii) or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of the date of the Securities Act prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; or |
| (ii) | If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use. |
(5) That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
(b) The undersigned registrant hereby undertakes that, for purposes of determining any liability under the Securities Act, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Exchange Act (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Exchange Act) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| - II-3 - |
(c) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
| - II-4 - |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-3 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Atlanta, State of Georgia, on this 2ND day of August, 2023.
| ALZAMEND NEURO, INC. | ||
| By: | /s/ Stephan Jackman | |
| Stephan Jackman | ||
| Chief Executive Officer (principal executive officer) | ||
| By: | /s/ David J. Katzoff | |
| David J. Katzoff | ||
| Chief Financial Officer (principal financial officer) |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, each director and officer whose signature appears below constitutes and appoints each of Stephan Jackman and David J. Katzoff, his true and lawful attorney-in-fact and agent, with full power of substitution and re-substitution, to sign in any and all capacities any and all amendments or post-effective amendments to this registration statement on Form S-3, and to sign any and all additional registration statements relating to the same offering of securities of the Registration Statement that are filed pursuant to Rule 462(b) of the Securities Act, and to file the same with all exhibits thereto and other documents in connection therewith with the Securities and Exchange Commission, granting such attorney-in-fact and agent full power and authority to do all such other acts and execute all such other documents as he may deem necessary or desirable in connection with the foregoing, as fully as the undersigned may or could do in person, hereby ratifying and confirming all that such attorney-in-fact and agent may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registrant Statement has been signed by the following persons in the capacities and on the dates indicated.
| Name | Title | Date | ||
|
By: /s/ Stephan Jackman Stephan Jackman |
Chief Executive Officer and Director (principal executive officer) |
August 2, 2023 | ||
|
By: /s/ David J. Katzoff David J. Katzoff |
Chief Financial Officer (principal financial and accounting officer) |
August 2, 2023 | ||
|
By: /s/ William B. Horne William B. Horne |
Chairman of the Board | August 2, 2023 | ||
|
By: /s/ Henry C.W. Nisser Henry C.W. Nisser |
Executive Vice President, General Counsel and Director |
August 2, 2023 | ||
|
By: /s/ Mark Gustafson Mark Gustafson |
Director | August 2, 2023 | ||
|
By: /s/ Lynne Fahey McGrath, M.P.H., Ph.D. Lynne Fahey McGrath, M.P.H., Ph.D. |
Director | August 2, 2023 | ||
|
By: /s/ Andrew H. Woo, M.D., Ph.D. Andrew H. Woo, M.D., Ph.D |
Director | August 2, 2023 | ||
|
By: /s/ Jeffrey Oram Jeffrey Oram |
Director | August 2, 2023 |
- II-5 -